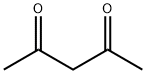
How to synthesize Acetylacetone?
Introduction
Acetylacetone is a commercial chemical with significant commercial value as a critical additive for aircraft fuel, metal extraction, and resin modification. It has been proven that acetylacetone could be obtained by ring opening and decarboxylation of Triacetic acid lactone (TAL) without any catalyst. Due to its broad application, TAL was selected as one of the ten most potential platform molecules by the US Department of Energy in 2004.
Production method
Acetylacetone has been prepared by the reaction of acetyl chloride with aluminum chloride, followed by hydrolysis; by the condensation of acetone with ethyl acetate in the presence of sodium, sodium amide, sodium ethoxide, and alkali or alkaline-earth hydrides; by the reaction of acetone and acetic anhydride in the presence of boron trifluoride; by the pyrolysis of isopropenyl acetate; by the reaction of ethyl acetoacetate and acetic anhydride in the presence of magnesium at 140°; from methyl or ethyl diacetylacetate by treatment with acids; and by the dehydrogenation of 4-pentanol-2-one in the presence of Raney nickel.
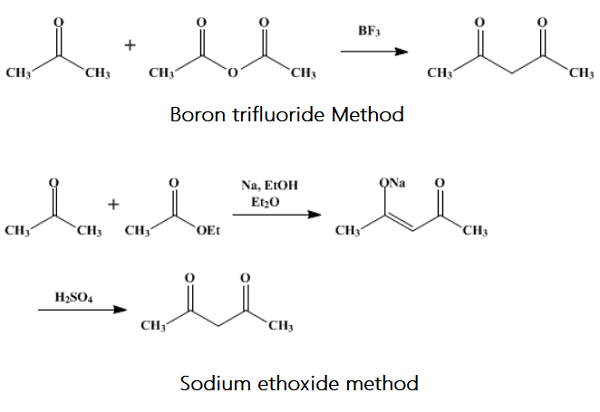
One hundred and sixteen grams (2 mol) of acetone and 510 g (5 mol) of reagent-grade acetic anhydride are placed in a 2-l three-necked flask and cooled in an ice-salt bath. One neck of the flask is stopped; the second one contains a tube for admitting boron trifluoride, and the third one contains an outlet tube leading to an alkali trap to catch any unabsorbed boron trifluoride. Commercial-grade boron trifluoride is passed through a Kjeldahl bulb to prevent the reaction mixture from sucking back into the cylinder. It is then bubbled into the reaction mixture at such a rate that 500 g is absorbed in about 5 hours (2 bubbles per second). The reaction mixture is poured into a solution of 800 g of hydrated sodium acetate in 1.6 l of water contained in a 5-l flask. The mixture is then steam-distilled, and the distillate is collected in the following portions: 1 l, 500 ml, 500 ml, 400 ml.
A solution of reagent-grade hydrated copper acetate is made by dissolving 240 g of the salt in 3 l of water at about 85℃ and filtering from any basic acetate. The copper salt of acetylacetone is then precipitated by adding 1.4 l of the hot copper acetate solution to the first fraction of the acetylacetone, 700 ml to the second, 500 ml to the third, and 400 ml to the fourth fraction. After standing for 3 hours, or better overnight, in a refrigerator, the salt is filtered, washed once with water, and sucked dry. The salt is shaken in a separatory funnel with 800 ml of 20% sulfuric acid and 800 ml of ether, and the ether layer is removed. The aqueous layer is extracted with 400 ml and then 200 ml of ether. The combined extracts are dried with 250 g of anhydrous sodium sulfate, and the ether is removed by distillation. The residue is distilled through a Widmer column, yielding 160–170 g of acetylacetone boiling at 134–136℃ (80–85% based on acetone).
You may like
Related articles And Qustion
See also
Lastest Price from Acetylacetone manufacturers

US $1.00/kg2025-04-21
- CAS:
- 123-54-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 123-54-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons