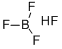Fluoroboric acid-Uses in Organic Analysis
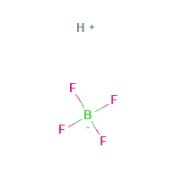
Fig 1. Chemical structure formula and three-dimensional structure of Fluoroboric acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula [H+][BF4-], where H+ represents the solvated proton. The solvent can be any suitably Lewis basic entity. For instance, in water, it can be represented by H3OBF4 (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: [H(H2O)n+][BF4-]. The ethyl ether solvate is also commercially available: [H(Et2O)n+][BF4-], where n is most likely.Unlike strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist.It is mainly produced as a precursor to other fluoroborate salts. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a weakly coordinating, non-oxidizing conjugate base. It is structurally similar to perchloric acid, but lacks the hazards associated with oxidants[1].
Pure HBF4 has been described as a "nonexistent compound", as a sufficiently 'naked' proton is expected to abstract a fluoride from the tetrafluoroborate ion to give hydrogen fluoride and boron trifluoride: [H+][BF4-] → HF + BF3. However, a solution of BF3 in HF is highly acidic, having an approximate speciation of [H2F+][BF4-] and a Hammett acidity function of -16.6 at 7 mol % BF3, easily qualifying as a superacid.Although the solvent-free HBF4 has not been isolated, its solvates are well characterized. These salts consist of protonated solvent as a cation, H3O+ and H5O2+, and the tetrahedral BF4- anion. The anion and cations are strongly hydrogen-bonded.Aqueous solutions of HBF4 are produced by dissolving boric acid in aqueous hydrofluoric acid.Three equivalents of HF react to give the intermediate boron trifluoride and the fourth gives fluoroboric acid:
B(OH)3 + 4 HF → H3O+ + BF4- + 2H2O
Anhydrous solutions can be prepared by treatment of aqueous fluoroboric acid with acetic anhydride[2-5].
Fluoroboric acid is the principal precursor to fluoroborate salts, which are typically prepared by treating the metal oxides with fluoroboric acid. The inorganic salts are intermediates in the manufacture of flame-retardant materials and glazing frits, and in electrolytic generation of boron. HBF4 is also used in aluminum etching and acid pickling[6].
References
[1]Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis.
[2]Reed, Christopher A. (2005). "Carborane acids. New "strong yet gentle" acids for organic and inorganic chemistry" (PDF). Chem. Commun. 0 (13): 1669–1677.
[3]Olah, George A.; Surya Prakash, G. K.; Sommer, Jean; Molnar, Arpad (2009-02-03). Superacid chemistry. Olah, George A. (George Andrew), 1927-2017,, Olah, George A. (George Andrew), 1927-2017.
[4]Mootz, D.; Steffen, M. "Crystal structures of acid hydrates and oxonium salts. XX. Oxonium tetrafluoroborates H3OBF4, [H5O2]BF4, and [H(CH3OH)2]BF4", Zeitschrift für Anorganische und Allgemeine Chemie 1981, vol. 482, pp. 193-200.
[5]https://pubchem.ncbi.nlm.nih.gov/compound/28118
[6]http://www.chemspider.com/Chemical-Structure.26156.html?rid=24eb4bbf-13af-4ba7-b123-922ce5cec288&page_num=0
You may like
Related articles And Qustion
See also
Lastest Price from Fluoroboric acid manufacturers

US $6.00/kg2024-01-08
- CAS:
- 16872-11-0
- Min. Order:
- 1kg
- Purity:
- 40.0% , 48%-50%
- Supply Ability:
- 5000ton

US $30.00-5.00/kg2023-12-11
- CAS:
- 16872-11-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons