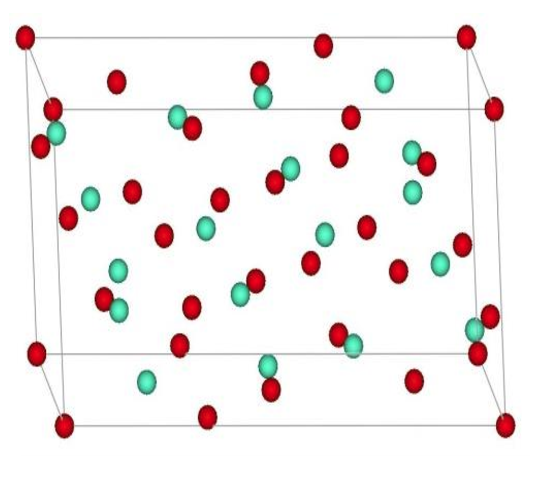
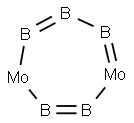Dimolybdenum pentaboride Crystal
Dimolybdenum pentaboride contain four to five boron atoms per each molybdenum atom. The estimated Vickers hardness of MoB5 is 37 to 39 GPa, which makes it a potential superhard material.
Dimolybdenum pentaboride has a wide homogeneity range: 66.7–70 at % boron. It is insoluble in water and has tm = 1600°C and ρ = 7.2 g/cm3. The best known process for the preparation of Mo2B5 is reaction between MoO3, boron carbide, and carbon black. In a vacuum from 10–2 to 10–1 mm Hg at temperatures from 1200 to 1300°C, this reaction reaches completion in 0.5°C 1 h.
Dimolybdenum pentaboride can also be prepared by heating a mixture of molybdenum and boron powders in a hydrogen atmosphere at a temperature from 1500 to 1600°C (2Mo + 5B = Mo2B5).
Yet another process for its synthesis is heating a mixture of molybdenum, boron oxide, and boron carbide in a hydrogen atmosphere at a temperature of 2000°C (28Mo + 5B2O3 + 15B4C = 14Mo2B5 + 15CO). It is worth noting that all of the existing industrial dimolybdenum pentaboride preparation processes require much energy and have low efficiency.