The structure of Molybdenum silicide
Introduction
Molybdenum, like other refractory metals, is characterized by a unique set of physical, mechanical, and chemical properties, which ensures its wide application in various fields. Molybdenum disilicide (MoSi2) is a promising candidate material for high-temperature structural applications. It is a high melting point (2030 °C) material with excellent oxidation resistance and a moderate density (6.24 g/cm3). However, low toughness at low temperatures and high creep rates at elevated temperatures have hindered its commercialization in structural applications. Much effort has been invested in MoSi2 composites as alternatives to pure molybdenum disilicide for oxidizing and aggressive environments[1].
Advantage
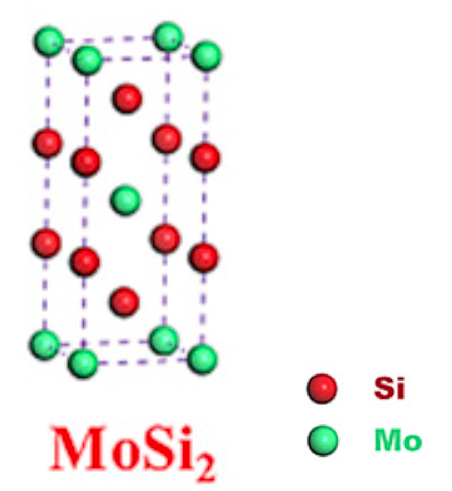
MoSi2 is the tetragonal α phase (C11b type) below 1900 °C, above which it transforms to the hexagonal β phase (C40 type). MoSi2 exhibits very excellent resistance to oxidation at elevated temperatures (up to 1700 °C), high thermal and electrical conductivity, relatively low density, good corrosion resistance, high ductile brittle-transition temperature as well as significant thermodynamic compatibility with many ceramic reinforcements[2]. These characteristics enable this material to be applied in a lot of fields, such as high-temperature structural materials, heating elements for furnaces, high-temperature oxidation-resistant coating, wear-resistant material, and reinforcement of composite material. Projected applications of MoSi2-based materials include turbine airfoils, combustion chamber components in oxidizing environments, missile nozzles, molten metal lances, industrial gas burners, diesel engine glow plugs, and materials for glass processing.
Structure
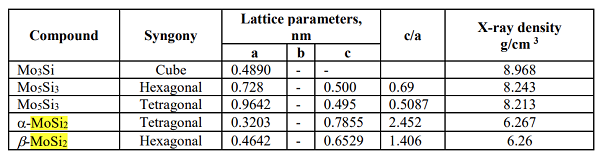
The MoSi2 compound melts at 2020 ± 20 °C; the homogeneity range is from 65.8 to 66.7 at. % silicon has a tetragonal structure. According to the study, the homogeneity region of MoSi2 obtained by diffusion saturation in a vacuum can be several percent and tends to increase with increasing siliconization temperature. The differences in the concentration of elements are: for Si = 2.52 ± 0.5%; for Mo = 2±0.5%. Silicide MoSi2 undergoes an allotropic transformation in the 1850-1900 °C temperature range. The low-temperature variety α-MoSi2 has a tetragonal structure. High temperature form β-MoSi2 has a hexagonal structure with parameters: a = 0.4642 ± 0.0005, c = 0.6529 ± 0.0005 nm, c/a - 1.406. The MoSi2 boundary on the Mo side is 67.1 ± 1.0% (at.). The low-temperature form of α-MoSi2 is a tetragonal cell with 2 Mo and 4 Si atoms. Si atoms form a frame, in the voids of which is Mo. The structure can also consist of layers parallel to the (010) plane with the closest hexagonal packing. The layers alternate in the order ABAB..., layer. B is displaced in the direction of the X-axis by a/2. The shortest Mo-Si distance is c/3. Chains of silicon atoms form zigzags passing through Mo prisms parallel to the X and Y axes.
References
[1] Z. Yao, T. S. Sudarshan, J. Stiglich. “Molybdenum silicide based materials and their properties.” Journal of Materials Engineering and Performance 8 3 (1999): 291–304.
[2] Guo-Dong Sun, Guo-Hua Zhang. “Study on the preparation of molybdenum silicides by the silicothermic reduction of MoS2.” Journal of Alloys and Compounds 728 (2017): Pages 295-306.
You may like
See also
Lastest Price from Molybdenum silicide manufacturers

US $0.00/KG2025-04-15
- CAS:
- 12136-78-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg