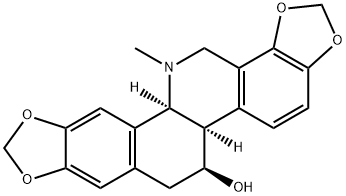
What is Chelidonine, and what can it be used for?
Chelidonine, also named ranunculine, is a benzophenanthridine alkaloid isolated from the whole plant of Chelidonium majus. L. It has been used as a painkiller in clinical treatment based on its morphine-like analgesic effect. Pharmacological researches in recent years have demonstrated that chelidonine played a role in antitumor, antibacterial, spasmolysis activities, and so on. And the antitumor activity is drawing more and more attention. However, its mechanisms of function remain unclear, which needs to be clarified in the future.
Chelidonine is an alkaloid fundamental parent, a benzophenanthridine alkaloid and an alkaloid antibiotic. Chelidonine is an isolate of Papaveraceae with acetylcholinesterase and butyrylcholinesterase inhibitory activity [1].
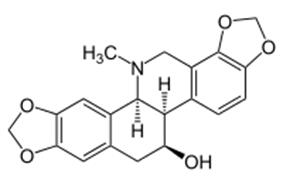
Chelidonine is the major alkaloid component of Chelidonium majus. Chelidonium majus L. is the only species of the tribe Chelidonieae of the Papaveraceae family. The Papaveraceae family is rich in specific alkaloids. C. majus contains various isoquinoline alkaloids with protopine, protoberberine and benzophenanthridine structures. This benzophenanthridine alkaloid can induce apoptosis in some transformed or malignant cell lines [2]. D-Chelidonine, the main alkaloid of Chelidonium majus, was first isolated in 1839. The supposed healing properties of greater celandine (Chelidonium majus) were believed in throughout Europe and Asia during the Emperial Roman period (Pliny 1966), and New World aboriginal cultures used BIA-containing plants by using sap or root extracts to treat minor cuts and infections [3].
Chelidonine is a major bioactive, isoquinoline alkaloid ingredient in Chelidonium majus. Benzylisoquinoline alkaloids (BIAs) are a structurally diverse group of plant specialized metabolites with a long history of investigation. A restricted number of enzyme families have been implicated in BIA metabolism. Whereas some enzymes exhibit a relatively broad substrate range, others are highly substrate specific. A small number of plant species, including opium poppy (Papaver somniferum) and other members of the Ranunculales, have emerged as model systems to study BIA metabolism. Recently, the emergence of transcriptomics, proteomics and metabolomics has expedited the discovery of new BIA biosynthetic genes.
Chelidonine is an isolate of Papaveraceae with acetylcholinesterase and butyrylcholinesterase (a nonspecific cholinesterase) inhibitory activity. AChE (acetylcholinesterase) inhibitors or anti-cholinesterases inhibit the cholinesterase enzyme from breaking down ACh, increasing both the level and duration of the neurotransmitter action. According to the mode of action, AChE inhibitors can be divided into two groups: irreversible and reversible. Reversible inhibitors, competitive or noncompetitive, mostly have therapeutic applications, while toxic effects are associated with irreversible AChE activity modulators. Reversible AChE inhibitors play an important role in pharmacological manipulation of the enzyme activity. These inhibitors include compounds with different functional groups (carbamate, quaternary or tertiary ammonium group), and have been applied in the diagnostic and/or treatment of various diseases such as: myasthenia gravis, AD, post-operative ileus, bladder distention, glaucoma, as well as antidote to anticholinergic overdose [4]. In general, methyltransferases of BIA metabolism accept a wide variety of alkaloid substrates with diverse backbone structures, with some showing more flexibility than others with respect to substrate range [3].
Chelidonine has been studied in multiple organisms, but mainly in rats and mice. In these organisms, sublethal doses of chelidonine caused ptosis tremor, sedation, and a decrease in body temperature. The LD50 of chelidonine, intraperitoneally administered, is in mice 1.3 g/kg and in rats 2 g/kg [1]. There are not many studies of toxicity of chelidonine in humans.
The greater celandine ‘Chelidonium majus’ and its main alkaloid chelidonine have previously been shown to exert high cytotoxicity against cancer cells. Furthermore, chelidonine is proposed to possess pro-apoptotic and anti-metastatic properties. Within the present study, the effects chelidonine on several HNSCC cell lines, as well as primary cells, were analyzed with respect to growth, migration, angiogenesis and apoptosis. Chelidonine suppressed the growth of all tested HNSCC cell lines, including a paclitaxel-resistant and P-glycoprotein (MDR1) overexpressing cell line, but not in a clear dose-dependent manner. Mucosal keratinocytes were also strongly affected by chelidonine, while fibroblasts proved to be much more resistant. Chelidonine failed to trigger apoptosis at physiological concentrations in HNSCC cell lines. Based on a spheroid invasion model chelidonine suppressed invasion of FaDu cells effectively on gelatin, fibronectin, collagen I, laminin and Matrigel®. However, invasion inhibition of the more aggressively invading cell line HLaC78 largely failed. Using the tube formation assay, chelidonine effectively inhibited angiogenesis. Expression analysis revealed an upregulation of the xenobiotic metabolism genes CYP1A1 and MDR1 by chelidonine. In summary, chelidonine appeared to exert only minor impact on head and neck cancer cells. Chelidonine did not produce clear dose-dependent and cell-type specific cytotoxicity nor did it trigger apoptosis strongly [5].
References
[1] https://en.wikipedia.org/wiki/Chelidonine
[2] Kemeny-Beke, A., Aradi, J., Beck, Z., Facsko, A., Berta, A., & Bodnar, A. (2006). Apoptotic response of uveal melanoma cells upon treatment with chelidonine, sanguinarine and chelerythrine. Elsevier, 237(1), 67-75.
[3] Hagel, J. M, & Facchini, P. J. (2013). Benzylisoquinoline Alkaloid Metabolism: A Century of Discovery and a Brave New World. Plant and Cell Physiology, 1(26), 4.
[4] Colovic, M. B. (2013). Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Current Neuropharmacology, 11(3), 315-335.
[5] RUTH HERRMANN, JEANETTE ROLLER, CHRISTINE POLEDNIK and MARIANNE SCHMIDT, Effect of chelidonine on growth, invasion, angiogenesis and gene expression in head and neck cancer cell lines, ONCOLOGY LETTERS 16: 3108-3116, 2018.
You may like
Related articles And Qustion
Lastest Price from CHELIDONINE manufacturers

US $1.00-1.00/Kg/Bag2024-02-01
- CAS:
- 476-32-4
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 50tons
US $0.00/mg2023-02-24
- CAS:
- 476-32-4
- Min. Order:
- 20mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g