Chelidonine: activities and toxicity
General Description
Chelidonine, derived from Chelidonium majus, demonstrates diverse pharmacological activities. It shows potential as an anti-cancer agent by targeting EGFR mutations and overcoming TKI resistance in NSCLC, presenting a promising therapeutic strategy. Additionally, chelidonine exhibits potent anti-inflammatory effects through the TLR4/NF-κB signaling pathway, suggesting its therapeutic value in combating inflammatory conditions. Furthermore, it has been studied for its antifungal properties, showing efficacy against Botryosphaeria dothidea and offering potential industrial applications. However, chelidonine also presents toxicity through inhibitory effects on cholinesterase enzymes, causing neurotoxicity and exerting various cytotoxic effects. Despite its promising pharmacological activities, the toxicity of chelidonine warrants careful consideration in potential clinical applications.

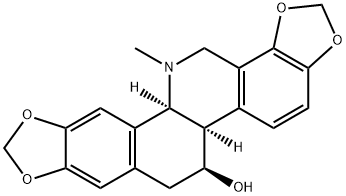
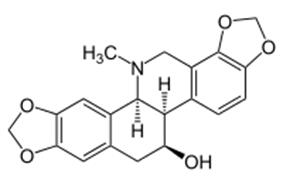
Figure 1. Chelidonine
Activities
Anti-cancer activities
Chelidonine has shown promising activities in inhibiting non-small cell lung cancer (NSCLC) with EGFR mutations and overcoming TKI resistance. It effectively suppresses the growth of NSCLC cells with EGFR double mutation by significantly inhibiting the mitochondrial respiratory chain. Furthermore, chelidonine was found to directly bind to EGFR, showing a higher binding affinity to EGFRL858R/T790M than EGFRWT, which selectively inhibits EGFR phosphorylation in cells with EGFR double mutation. Proteomics analysis revealed that chelidonine-induced apoptosis could be blocked by an inhibitor of AMPK. In vivo studies demonstrated that chelidonine has a similar inhibitory effect to the second-generation TKI Afatinib. Therefore, targeting EGFR and inhibiting mitochondrial function with chelidonine presents a promising therapeutic strategy for inhibiting NSCLC with EGFR mutation and TKI resistance. These findings suggest that chelidonine may serve as a potential new agent for the clinical treatment of NSCLC patients with EGFR mutations, addressing the issue of drug resistance. 1
Anti-inflammatory activity
Chelidonine exhibits potent anti-inflammatory activity through multiple molecular mechanisms. In both in vitro and in vivo studies using RAW264.7 macrophages and mice, chelidonine was found to effectively suppress the LPS-induced inflammatory response. It significantly inhibited the production of NO and PGE2, as well as the expression of iNOS and COX-2 at both mRNA and protein levels. Additionally, chelidonine attenuated the release of pro-inflammatory cytokines TNFα and IL-6 induced by LPS. Mechanistically, chelidonine disrupted the TLR4/NF-κB signaling pathway by inhibiting the activation and degradation of IκBα, as well as the translocation of p65 from the cytoplasm to the nucleus. Furthermore, chelidonine notably reduced the expression of TLR4 induced by LPS. In LPS-stimulated mice, chelidonine significantly decreased serum TNFα, IL-6, and PGE2 levels. These findings collectively demonstrate that chelidonine exerts anti-inflammatory effects by targeting the TLR4/NF-κB signaling pathway, suggesting its potential therapeutic value in combating inflammatory conditions. 2
Anti-fungal activity
Chelidonine has been studied for its antifungal properties. Researchers purified chelidonine from C. majus L. using D101 macroporous adsorption resin, achieving a 14.16-fold increase in concentration with an 80.77% recovery yield. The antifungal activity of enriched chelidonine against Botryosphaeria dothidea was evaluated, revealing EC50 values of 0.43mg/mL for enriched chelidonine products and 0.77mg/mL for chelidonine standard, indicating its efficacy as an antifungal agent. The study utilized pseudo-second-order kinetics and Freundlich equation models to characterize the adsorption processes on D101 resin. Additionally, the method of chelidonine enrichment was deemed highly efficient, cost-effective, and environmentally friendly, suggesting its potential for industrial applications. Overall, the findings demonstrate the promising antifungal activity of chelidonine and highlight a practical and sustainable approach for its purification, underlining its potential significance in the field of herbal medicine. 3
Toxicity
Chelidonine, found in Chelidonium majus, exhibits toxicity through its inhibitory effects on cholinesterase enzymes. By suppressing acetylcholinesterase (AChE), it disrupts the breakdown of acetylcholine, leading to the continuous transmission of nerve impulses and sustained muscle contractions. This neurotoxicity can result in symptoms such as excessive salivation, eye-watering, muscle spasms, and even death. Substances like nerve gases and insecticides act as AChE inhibitors by binding to the active site of the enzyme. Chelidonine, specifically, inhibits both acetylcholinesterase and butyrylcholinesterase. Additionally, it intercalates DNA, inhibits DNA and RNA polymerase, topoisomerase, telomerase, and ribosomal protein biosynthesis, and binds to tubulin/microtubules, acting as spindle poisons. Chelidonine has also demonstrated the ability to overcome multidrug resistance in cancer cells by interacting with ABC-transporters, CYP3A4, and GST enzymes, inducing apoptosis, and exerting cytotoxic effects. It has been shown to cause mitotic arrest and exhibit weak inhibitory effects on cell growth across various cell lines. 4
Reference
1. Xie YJ, Gao WN, Wu QB, Yao XJ, Jiang ZB, Wang YW, Wang WJ, Li W, Hussain S, Liu L, Leung EL, Fan XX. Chelidonine selectively inhibits the growth of gefitinib-resistant non-small cell lung cancer cells through the EGFR-AMPK pathway. Pharmacol Res. 2020 Sep;159:104934.
2. Liao W, He X, Yi Z, Xiang W, Ding Y. Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264.7 macrophages. Biomed Pharmacother. 2018 Nov;107:1151-1159.
3. Pan J, Yang Y, Zhang R, Yao H, Ge K, Zhang M, Ma L. Enrichment of chelidonine from Chelidonium majus L. using macroporous resin and its antifungal activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Dec 1;1070:7-14.
4. Chelidonine. Toxin and Toxin Target Database, Accession Number: T3D4087.
Related articles And Qustion
See also
Lastest Price from CHELIDONINE manufacturers

US $0.00/kg2025-05-07
- CAS:
- 476-32-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg
US $0.00/mg2023-02-24
- CAS:
- 476-32-4
- Min. Order:
- 20mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g