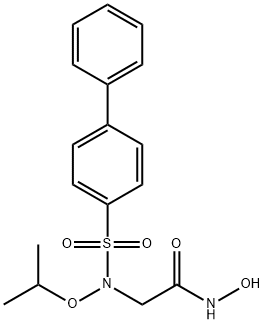
Matrix metalloproteinases (MMPs) belong to a family of proteases that play a crucial role in tissue remodeling and repair by degrading extracellular matrix proteins to enable cell migration. During cancer progression both MMP-2 and MMP-9 play a role in metastatic tumor dispersion and angiogenesis. ARP 100 is a biphenylsulfonamide that acts as a selective inhibitor of MMP-2 demonstrating an IC50 value of 12 nM. Due to its specific zinc binding domain configuration, the inhibitory activity of ARP 100 is significantly less potent towards MMP-1, MMP-3, MMP-7, and MMP-9 (IC50 values are 50, 4.5, 50, and 2 μM, respectively). At 50 nM, ARP 100 suppresses the invasive behavior of HT1080 tumor cells grown on matrigel.