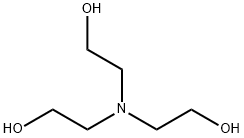
Триэтаноламин
- английское имяTriethanolamine
- CAS №102-71-6
- CBNumberCB9852620
- ФормулаC6H15NO3
- мольный вес149.19
- EINECS203-049-8
- номер MDLMFCD00002855
- файл Mol102-71-6.mol
| Температура плавления | 17.9-21 °C (lit.) |
| Температура кипения | 190-193 °C/5 mmHg (lit.) |
| плотность | 1.124 g/mL at 25 °C (lit.) |
| плотность пара | 5.14 (vs air) |
| давление пара | 0.01 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 365 °F |
| температура хранения | Store at RT. |
| растворимость | H2O: 1 M, clear, colorless |
| форма | Oily Liquid |
| Удельный вес | 1.125 (20/20℃) |
| цвет | Clear colorless to slightly yellow |
| РН | 10.5-11.5 (25℃, 1M in H2O) |
| пка | 7.8(at 25℃) |
| Водородный показатель | 7.3 - 8.3 |
| Запах | Mild ammoniacal. |
| Пределы взрываемости | 3.6-7.2%(V) |
| Растворимость в воде | soluble |
| Чувствительный | Air Sensitive & Hygroscopic |
| λмакс | λ: 280 nm Amax: 0.1 |
| Мерк | 14,9665 |
| БРН | 1699263 |
| Пределы воздействия | ACGIH: TWA 5 mg/m3 |
| Диэлектрическая постоянная | 6.9(40℃) |
| ИнЧИКей | GSEJCLTVZPLZKY-UHFFFAOYSA-N |
| LogP | -2.3 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | TRIETHANOLAMINE |
| FDA 21 CFR | 173.315; 175.105; 175.300; 176.170; 176.180; 176.200; 177.2600; 177.2800; 178.3910; 310.545; 352.70 |
| Справочник по базе данных CAS | 102-71-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-5 |
| FDA UNII | 9O3K93S3TK |
| Код УВД | D03AX12 |
| Справочник по химии NIST | Triethanolamine(102-71-6) |
| МАИР | 3 (Vol. 77) 2000 |
| Система регистрации веществ EPA | Triethanolamine (102-71-6) |
| Информация о косметике | Triethanolamine |
| UNSPSC Code | 12352116 |
| NACRES | NB.21 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38-36 | |||||||||
| Заявления о безопасности | 26-39-36 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | KL9275000 | |||||||||
| F | 3-10-23 | |||||||||
| Температура самовоспламенения | 600 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29221310 | |||||||||
| кода HS | 29321900 | |||||||||
| Банк данных об опасных веществах | 102-71-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Триэтаноламин химические свойства, назначение, производство
Описание
Triethanolamine is a viscous, colourless/pale yellow liquid with a weak ammoniacal odour. Triethanolamine is incompatible with copper, copper alloys, galvanised iron, acids, and oxidisers. Reports indicate that in India itself, as many as six companies manufacture triethanolamine and it is manufactured by many different countries around the world. Global production and industrial application of triethanolamine is very extensive.
In industries, triethanolamine is used as a corrosion inhibitor in metal-cutting fluids; a curing agent for epoxy and rubber polymers; a copper–triethanolamine; in emulsifiers, thickeners, and wetting agents in the formulation of consumer products such as cosmetics, detergents, shampoos, and other personal products; and a neutraliser-dispersing agent in agricultural herbicide formulations. In brief, triethanolamine has wide applications as a corrosion inhibitor, a surface-active agent, and an intermediate in various products including metalworking fluids, oils, fuels, paints, inks, cement, cosmetic, and personal products and formulations of algicides and herbicides.
Химические свойства
Triethanolamine is a pale yellow and viscous liquid. It is hygroscopic with an irritant and ammoniacal odor. There are multiple industrial and domestic applications for this compound, i.e., in the manufacture of toilet products, cosmetics formulations, solvents for waxes, resins, dyes, paraffi ns and polishes, herbicides, and lubricants for textile products. In the pharmaceutical industry, triethanolamine is used as a non-steroidal, antiinfl ammatory agent, an emulsifi er, and an alkylating agent.Использование
Triethanolamine is used primarily as a surfactant, reducing the surface tension between two media. It is also used as a general emulsifier for preparations, such as ones involving drug penetration ass ays.Методы производства
Triethanolamine is produced with ethanolamine and diethanolamine by ammonolysis of ethylene oxide and the triethanolamine is then separated by distillation (Mullins 1978). In 1984, 139.6 million pounds of triethanolamine were produced in the United States (USTIC 1985).Определение
ChEBI: Triethanolamine is a tertiary amino compound that is ammonia in which each of the hydrogens is substituted by a 2-hydroxyethyl group. It has a role as a buffer and a surfactant. It is a tertiary amino compound, a triol and an amino alcohol. It is functionally related to a triethylamine. It is a conjugate base of a triethanolammonium.Подготовка
Triethanolamine is prepared commercially by the ammonolysis of ethylene oxide. The reaction yields a mixture of monoethanolamine, diethanolamine, and triethanolamine, which are separated to obtain the pure products.Всемирная организация здравоохранения(ВОЗ)
Trolamine is widely used as an emulsifier in combination with fatty acids in pharmaceutical and cosmetic products. The World Health Organization is not aware of restrictive action having been taken elsewhere.Общее описание
Oily liquid with a mild ammonia odor. Denser than water. Freezing point is 71°F.Реакции воздуха и воды
Water soluble.Профиль реактивности
Triethanolamine is an aminoalcohol. Neutralize acids to form salts plus water in exothermic reactions. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Reacts violently with strong oxidants. [Handling Chemicals Safely 1980. p. 928].Угроза здоровью
Exposures to triethanolamine, in contrast with other chemical compounds, is known to cause low toxicity to animals and the acute oral LD50 to rats and guinea pigs ranges from 8000 to 9000 mg/kg. Triethanolamine was found to be a moderate eye irritant. A 5%–10% solution of triethanolamine did not induce skin irritation or skin sensitization. Studies of Inoue et al. and many other workers have indicated the absence of the mutagenic potential of triethanolamine as evidenced by both in vivo and in vitro studies (Salmonella typhimurium tests, Chinese hamster ovary cells, and rat liver chromosome analysis). Further, extensive studies have demonstrated the absence of potential carcinogenicity of triethanolamine in rats and mice, suggesting a low or lack of acute or chronic toxicity of the chemical to mammals.Пожароопасность
Special Hazards of Combustion Products: Poisonous gases, such as NOx, may be producedХимическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Фармацевтические приложения
Triethanolamine is widely used in topical pharmaceutical formulations, primarily in the formation of emulsions.When mixed in equimolar proportions with a fatty acid, such as stearic acid or oleic acid, triethanolamine forms an anionic soap with a pH of about 8, which may be used as an emulsifying agent to produce fine-grained, stable oil-in-water emulsions. Concentrations that are typically used for emulsification are 2–4% v/v of triethanolamine and 2–5 times that of fatty acids. In the case of mineral oils, 5% v/v of triethanolamine will be needed, with an appropriate increase in the amount of fatty acid used. Preparations that contain triethanolamine soaps tend to darken on storage. However, discoloration may be reduced by avoiding exposure to light and contact with metals and metal ions.
Triethanolamine is also used in salt formation for injectable solutions and in topical analgesic preparations. It is also used in sun screen preparations.
Triethanolamine is used as an intermediate in the manufacturing of surfactants, textile specialties, waxes, polishes, herbicides, petroleum demulsifiers, toilet goods, cement additives, and cutting oils. Triethanolamine is also claimed to be used for the production of lubricants for the rubber gloves and textile industries. Other general uses are as buffers, solvents, and polymer plasticizers, and as a humectant.
Контактные аллергены
This emulsifying agent can be contained in many products such as cosmetics, topical medicines, metalworking cut- ting fluids, and color film developers. Traces may exist in other ethanolamines such as monoand diethanolamine. Contact allergy seems to be rarer than previously thought.Профиль безопасности
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Liver and kidney damage have been demonstrated in animals from chronic exposure. A human and experimental skin irritant. An eye irritant. Questionable carcinogen with experimental carcinogenic data. Combustible liquid when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx and CN-.Безопасность
Triethanolamine is used primarily as an emulsifying agent in a variety of topical pharmaceutical preparations. Although generally regarded as a nontoxic material, triethanolamine may cause hypersensitivity or be irritant to the skin when present in formulated products. The lethal human oral dose of triethanolamine is estimated to be 5–15 g/kg body-weight.Following concern about the possible production of nitrosamines in the stomach, the Swiss authorities have restricted the use of triethanolamine to preparations intended for external use.
LD50 (guinea pig, oral): 5.3 g/kg
LD50 (mouse, IP): 1.45 g/kg
LD50 (mouse, oral): 7.4 g/kg
LD50 (rat, oral): 8 g/kg
Возможный контакт
Monoethanolamine is widely used in industry for scrubbing acid gases and in production of detergents and alkanolamide surfactants; to remove carbon dioxide and hydrogen from natural gas, to remove hydrogen sulfide and carbonyl sulfide; as an alkaline conditioning agent; as an intermediate for soaps, detergents, dyes, and textile agents. Diethanolamine is an absorbent for gases; a solubilizer for 2,4- dichlorophenoxyacetic acid (2,4-D); and a softener and emulsifier intermediate for detergents. It also finds use in the dye and textile industry. Triethanolamine is used as plasticizers, neutralizer for alkaline dispersions; lubricant additive; corrosion inhibitor; and in the manufacture of soaps, detergents, shampoos, shaving preparations; face and hand creams; cements, cutting oils, insecticides, surface active agents; waxes, polishes, and herbicides.Канцерогенность
Results of carcinogenicity studies have been controversial. Hoshino and Tanooka reported that triethanolamine in the diet of mice at levels of 0.03% or 0.3% caused a significant increase in the occurrence of tumors, both benign and malignant. Females showed a 32% increase, mostly of thymic lymphomas. The increase of all other tumors, in both sexes, was 8.2%. They also found that triethanolamine reacted with sodium nitrite to produce N-nitrosodiethanolamine and that the product caused mutagenesis in bacteria. Maekawa et al. reported that no carcinogenic activity was found when given orally to rats in drinking water at concentrations of 1% and 2% for 2 years. However, the dosage to females was halved after week 69 of treatment owing to nephrotoxicity. Histological examination of renal damage in treated animals revealed acceleration of chronic nephropathy, mineralization of the renal papilla, nodular hyperplasia of the pelvic mucosa, and pyelonephritis with or without papillary necrosis. Nephrotoxicity seemed to affect life span adversely, especially in females. Tumor incidence and histology were the same in the treated group as in controls.хранилище
Triethanolamine may turn brown on exposure to air and light.The 85% grade of triethanolamine tends to stratify below 15℃; homegeneity can be restored by warming and mixing before use. Triethanolamine should be stored in an airtight container protected from light, in a cool, dry place.
See Monoethanolamine for further information.
Перевозки
UN2491 Ethanol amine or Ethanolamine solutions, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Shake the amine gently with Linde type 4A molecular sieves for 24hours, filter and fractionate it under a vacuum, and preferably in the presence of N2. Store it in dark stoppered bottles under N2 as it is hygroscopic, and turns brown in air and light. It has a strong ammoniacal odour (like diethanolamine). It is miscible with H2O, MeOH and Me2CO, and its solubilities at 25o in n-heptane, Et2O and *C6H6 are 0.4%, 1.6% and 4.2%, respectively. [See diethanolamine above, Beilstein 4 IV 1524.]Несовместимости
Triethanolamine is a tertiary amine that contains hydroxy groups; it is capable of undergoing reactions typical of tertiary amines and alcohols. Triethanolamine will react with mineral acids to form crystalline salts and esters. With the higher fatty acids, triethanolamine forms salts that are soluble in water and have characteristics of soaps. Triethanolamine will also react with copper to form complex salts. Discoloration and precipitation can take place in the presence of heavy metal salts.Triethanolamine can react with reagents such as thionyl chloride to replace the hydroxy groups with halogens. The products of these reactions are very toxic, resembling other nitrogen mustards.
Утилизация отходов
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissionsРегуляторный статус
Included in the FDA Inactive Ingredients Database (rectal, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Триэтаноламин запасные части и сырье
сырьё
1of2
запасной предмет
- antistatic Agent P
- 3-хлорпиразин-2-карбoнитрил
- alcohol polyoxyethylene ether phosphoric monoester ethanolamine
- lauryl polyoxyethylene ether triethanol amine salt
- dodecyl phenyl ammonium sulfate
- cationic fatliquor agent Z-2
- LY 171883
- 2-[bis(2-hydroxyethyl)amino]ethyl stearate
- Water treatment agent POE
- Concrete antifreeze
- Triethanolamine borate
- Cutting liquor,synthetic
- hydrogen peroxide stabilizer C-75
- ribonucleic acid for injection
- золь пятиокиси сурьмы
- polyurethae finishes PUC series
- antistatic Agent TM
- Antifoaming agent DEF
- 1,4-бис(трихлорметил)бензол
- NS-01 silicone modified polyurethane water proof an dluster agent
- tri-isopropanlamiue polyoxypropyleal polyoxy-ethylene ether
- Spining lubricant agent
- nonylphenyl polyoxyethylene ether sulfate triethanolamine
- Antifreeze
- Sulfonated oil DAH
1of8
Триэтаноламин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +8619931165850 | China | 1000 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-0532-86867058 +86-18562606086 |
China | 478 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +8615350851019 | China | 1001 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |