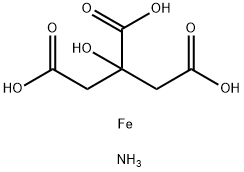
Аммоний железа(III) цитрат
- английское имяAmmonium ferric citrate
- CAS №1185-57-5
- CBNumberCB9729858
- ФормулаC6H11FeNO7
- мольный вес265
- EINECS214-686-6
- номер MDLMFCD00013099
- файл Mol1185-57-5.mol
химическое свойство
| Температура плавления | >193°C (dec.) |
| Температура кипения | 498℃[at 101 325 Pa] |
| плотность | 1.06[at 20℃] |
| давление пара | 0Pa at 25℃ |
| температура хранения | Store at RT. |
| растворимость | 1200g/l |
| форма | powder |
| цвет | Green |
| Запах | Odorless to slight ammonia odor |
| РН | 5.0~8.0 |
| Биологические источники | synthetic |
| Растворимость в воде | 1200 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| Мерк | 14,4017 |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Стабильность | Hygroscopic |
| LogP | -0.737 at 25℃ |
| Справочник по базе данных CAS | 1185-57-5(CAS DataBase Reference) |
| FDA 21 CFR | 184.1296; 73.1025; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | IRON AMMONIUM CITRATE |
| Специальный комитет по веществам GRAS | Ferric ammonium citrate |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | UVP74NG1C5 |
| Код УВД | V08CA07 |
| Система регистрации веществ EPA | Ferric ammonium citrate (1185-57-5) |
| UNSPSC Code | 41171614 |
| NACRES | NA.74 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| РИДАДР | UN 9118 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GE7540000 | |||||||||
| F | 3-8 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29181500 | |||||||||
| Банк данных об опасных веществах | 1185-57-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Аммоний железа(III) цитрат химические свойства, назначение, производство
Описание
Ammonium ferric citrate is a food additive with E number E381 used as an acidity regulator. It is a green or reddish-brown powder which is very soluble in water.The molecular formula of ammonium iron (III) citrate is variable. It can be prepared by adding Fe(OH)3 to an aqueous solution of citric acid and ammonia . The brown form is approximately 9% NH3, 16.5 – 18.5 % Fe , and 65 % hydrated citric acid; the green form is approximately 7.5 % NH3 , 14.5 – 16 % Fe, and 75% hydrated citric acid. The green type is more readily reduced by light than the brown.
Other uses for ammonium ferric citrate include water purification and printing (cyano type). It is used as a reducing agent of metal salts of low activity like gold and silver and is also in a commonly used recipe with potassium ferricyanide to make cyanotype prints. Ammonium ferric citrate is also used in Kligler iron deeps to determine hydrogen sulfide production in microbial metabolism.
In medicine, ammonium ferric citrate is used as a contrast medium. It is also used as a hematinic.
Химические свойства
brownish-yellow to brown powderИспользование
HematinicОбщее описание
Ammonium ferric citrate is a yellowish brown to red solid with a faint odor of ammonia. Ammonium ferric citrate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium ferric citrate is used in medicine, in making blueprints, and as a feed additive.Реакции воздуха и воды
Water soluble.Профиль реактивности
Acidic salts, such as Ammonium ferric citrate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires [USCG, 1999].Угроза здоровью
Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and causes mild irritation of skin on prolonged contact.Пожароопасность
Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires.Возможный контакт
Ferric ammonium citrate is used in blueprinting, photography, medical treatment; and as an animal food additive.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Несовместимости
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).Аммоний железа(III) цитрат запасные части и сырье
сырьё
1of2
запасной предмет
Аммоний железа(III) цитрат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618062705058 | China | 140 | 58 | ||
| +8617732866630 | China | 8773 | 58 | ||
| +8613288715578 | China | 12554 | 58 | ||
| +86-13131129325 | China | 5251 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1001 | 58 | ||
| +86-17331933971 | China | 2472 | 58 | ||
| +86-15350851019; +8615350851019 |
China | 1000 | 58 | ||
| +86-16264648883 | China | 7245 | 58 | ||
| +86-0086-19851820538 +8619851820538 |
China | 89 | 58 | ||
| +86-2158073036 | China | 12881 | 58 |