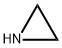
ЭТИЛЕНИМИН
- английское имяEthyleneimine
- CAS №151-56-4
- CBNumberCB9489965
- ФормулаC2H5N
- мольный вес43.07
- EINECS205-793-9
- номер MDLMFCD00039669
- файл Mol151-56-4.mol
| Температура плавления | -78°C |
| Температура кипения | 56°C |
| плотность | 0,83 g/cm3 |
| давление пара | 160 at 20 °C, 250 at 30 °C (quoted, Verschueren, 1983) |
| показатель преломления | nD25 1.412 |
| Fp | -11°C |
| растворимость | miscible with water and virtually all organic solvents |
| форма | Colorless liquid |
| пка | 8.01(at 25℃) |
| Запах | Fishy; ammoniacal. |
| Растворимость в воде | miscible |
| констант закона Генри | 1.33(x 10-7 atm?m3/mol) at 25 °C (quoted, Mercer et al., 1990) |
| Диэлектрическая постоянная | 18.3(25℃) |
| Пределы воздействия | TLV-TWA (skin) 0.5 ppm (~1 mg/m3) (ACGIH, OSHA, and MSHA); Poten tial Human Carcinogen in the workplace (OSHA), Potential Carcinogen (NIOSH). |
| Стабильность | Highly flammable. Reacts with a wide variety of materials. |
| LogP | -2.68--0.28 at 25℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | ETHYLENIMINE |
| Справочник по базе данных CAS | 151-56-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6 |
| FDA UNII | 54P5FEX9FH |
| Предложение 65 Список | Ethyleneimine |
| Справочник по химии NIST | Ethylenimine(151-56-4) |
| МАИР | 2B (Vol. 9, Sup 7, 71) 1999 |
| Система регистрации веществ EPA | Aziridine (151-56-4) |
| Коды опасности | F;T,T,F,N,T+ | |||||||||
| Заявления о рисках | 11-26/27/28-34-45-46-51/53 | |||||||||
| Заявления о безопасности | 45-53-61 | |||||||||
| РИДАДР | 1185 | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| Банк данных об опасных веществах | 151-56-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 15 mg/kg (Smyth) | |||||||||
| ИДЛА | 100 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H411:Токсично для водных организмов с долгосрочными последствиями.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
H310:Смертельно при попадании на кожу.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H300:Смертельно при проглатывании.
H340:Может вызывать генетические дефекты.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P242:Использовать неискрящие приборы.
P243:Принимать меры предосторожности против статических разрядов.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P262:Избегать попадания в глаза, на кожу или одежду.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P302+P350:ПРИ ПОПАДАНИИ НА КОЖУ: Осторожно промыть большим количеством воды с мылом.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P403+P235:Хранить в прохладном, хорошо вентилируемом месте.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ЭТИЛЕНИМИН химические свойства, назначение, производство
Описание
Ethyleneimine is a colourless liquid with an ammonia-like smell or pungent odour. It is highly flammable and reacts with a wide variety of materials. Ethyleneimine is used in polymerisation products, as a monomer for polyethyleneimine and as a comonomer for polymers, for example, with ethylenediamine. Polymerised ethyleneimine is used in paper, textile chemicals, adhesive binders, petroleum, refining chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid flocculants, and surfactants. Ethyleneimine readily polymerises, and it behaves like a secondary amine. Ethyleneimine is highly caustic, attacking materials such as cork, rubber, many plastics, metals, and glass except those without carbonate or borax. It polymerises explosively on contact with silver, aluminium, or acid. The activity of ethyleneimine is similar to that of nitrogen and sulphur mustards. Ethyleneimine is used as an intermediate in the production of triethylenemelamine.Химические свойства
Ethyleneimine is a colorless liquid with an ammonia-like smell or pungent odor. It is highly flammable and reacts with a wide variety of materials. Ethyleneimine is used in polymerization products, as a monomer for polyethyleneimin, and as a comonomer for polymers, e.g., with ethylenediamine. Polymerized ethylenimine is used in paper, textile chemicals, adhesive binders, petroleum, refi ning chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid fl occulants, and surfactants. Ethyleneimine readily polymerizes, and it behaves like a secondary amine. Ethyleneimine is highly caustic, attacking materials such as cork, rubber, many plastics, metals, and glas except those without carbonate or borax. It polymerizes explosively on contact with silver, aluminum, or acid. The activity of ethyleneimine is similar to that of nitrogen and sulfur mustards. Ethyleneimine is used as an intermediate in the production of triethylenemelamine. Polymerized ethyleneimine is used in paper, textile chemicals, adhesive binders, petroleum, refi ning chemicals, fuels, lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid fl occulants, and surfactantsФизические свойства
Clear, colorless, very flammable liquid with a very strong ammonia odor. Odor threshold concentration is 1.5 ppm (quoted, Amoore and Hautala, 1983).Использование
Ethyleneimine is used to manufacture triethylenemelamine and is used in its polymeric form in paper and textile chemicals, adhesive binders, petroleum-refining chemicals, fuels and lubricants, coating resins, varnishes, lacquers, agricultural chemicals, cosmetics, ion-exchange resins, photographic chemicals, colloid flocculants, and surfactants.Методы производства
Industrial quantities are made with monoethanolamine via a two-step chemical dehydration process using sulphuric acid and sodium hydroxide, or by reacting 1,2-dichloroethane with ammonia. The U.S. production in 1978 was over 1500 metric tons (Ham 1978).Origin
Ethylenimine was first prepared in 1888 by GABRIEL, who mistakenly called it vinylamine. He prepared the ethylenimine by reacting 2-bromoethylamine hydrobromide with silver oxide or potassium hydroxide.Общее описание
A clear colorless liquid with an ammonia-like odor. Flash point 12°F. Less dense than water. Flammable over a wide range of vapor-air concentrations. Vapors irritate the skin, eyes, nose, and throat. May be toxic by prolonged inhalation, skin absorption, or ingestion. Carcinogenic. Vapors heavier than air. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently.Реакции воздуха и воды
Highly flammable. Soluble in water.Профиль реактивности
ETHYLENEIMINE vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents. Produces toxic oxides of nitrogen during combustion. Reacts with sodium hypochlorite and other chlorinating agents to give the explosive compound 1-chloroazidine. Decomposes if heated under pressure. or else hazardous polymerization may occur. Incompatible with silver or aluminum, which induce polymerization May polymerize explosively upon contact with acids. Polymerization is catalyzed by carbon dioxide [EPA, 1998].Опасность
Corrosive, absorbed by skin, causes tumors; exposure should be minimized; a carcinogen. Dangerous fire and explosion hazard, flammable limits in air 3.6–46%. Toxic by skin absorption; possible carcinogen.Угроза здоровью
Ethyleneimine is classified as extremely toxic with a probable oral lethal dose of 5-50 mg/kg which is approximately 7 drops to 1 teaspoonful for a 70 kg (150 lb.) person. Ethyleneimine gives inadequate warning when over-exposure is by inhalation or skin absorption. It is a severe blistering agent, causing third degree chemical burns of the skin. Also, it has a corrosive effect on mucous membranes and may cause scarring of the esophagus. It is corrosive to eye tissue and may cause permanent corneal opacity and conjunctival scarring. Severe exposure may result in overwhelming pulmonary edema. Renal damage has been described. Hemorrhagic congestion of all internal organs has been observed.Пожароопасность
Irritating vapors are generated when heated. Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. May polymerize in fires with evolution of heat and container rupture. Runoff to sewer may create fire or explosion hazard. Ethyleneimine vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents. Toxic oxides of nitrogen are produced during combustion. Upon treatment with sodium hypochlorite, Ethyleneimine gives off the explosive compound 1-chloroazidine. Avoid acids, sodium hypochlorite. If heated under pressure, instability may result. Hazardous polymerization may occur. Avoid contact with silver or aluminum. Explosive polymerization may occur upon contact with acids. Polymerization is catalyzed by carbon dioxide.Химическая реактивность
Reactivity with Water: Mild reaction, non-hazardous; Reactivity with Common Materials: Contact with silver or aluminum may cause polymerization; Stability During Transport: Stable unless heated under pressure; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Explosive polymerization can occur when in contact with acids; Inhibitor of Polymerization: None used.Промышленное использование
Approximately 50% of ethylenimine produced in the U.S. is polymerized to polyethyleneimine, used as a flocculant in water treatment, and as a wet-strength additive in the textile and paper industries. Polyethylenimine is also used in various adhesives and coatings and to laminate plastic films to paper, other cellulose materials, and metal foils for making cartons in the food industry. The adhesion properties of acrylic latex paints are improved by reaction of acid groups with ethylenimine. Ethylenimines are utilized in the textile industry to improve durability, crease resistance, flame resistance, and dyeing properties. Other uses are found in ion-exchange resin synthesis, in electroplating, as a rocket propellant binder, as a lubricating oil dispersant, and as a hardening agent in the preparation of photographic films. Ethylenimine is used in the manufacture of triethylene melamine, a cancer chemotherapy drug; various ethylenimines are used as insect chemosterilant agents for pest control (Ham 1978).Профиль безопасности
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Other experimental reproductive effects. Poison by ingestion, skin contact, inhalation, and intraperitoneal routes. Human mutation data reported. A skin, mucous membrane, and severe eye irritant. An allergc sensitizer of skin. Causes opaque cornea, keratoconus, and necrosis of cornea (experimentally). Has been known to cause severe human eye injury. Drinking of carbonated beverages is recommended as an antidote to ths material in stomach. A very dangerous fire and explosion hazard when exposed to heat, flame, or oxidzers. Reacts violently with acids, aluminum chloride + substituted anilines, acetic acid, acetic anhydride, acrolein, acrylic acid, allyl chloride, CS2, Cl2, chlorosulfonic acid, epichlorohydrin, glyoxal, HCl, HF, HNO3, oleum, P-propiolactone, Ag, NaOCl, H2SO4, vinyl acetate. Reacts with chlorinating agents (e.g., sodum hypochlorite solution) to form the explosive 1 chloroaziridine. Reacts with silver or its alloys to form explosive silver derivatives. Dangerous; heat and/or the presence of catalytically active metals or chloride ions can cause a violent exothermic reaction. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Ethyleneimine is used in production of binding agents; formation of plastics; and improving paper strength; in many organic syntheses; as an intermediate and monomer for fuel oil and lubricating refining. The polymerization products, polyethyleneimines, are used as auxiliaries in the paper industry and as flocculation aids in the clarification of effluents. It is also used in the textile industry for increasing wet strength, flame-, water-, shrinkproofing, and stiffeningКанцерогенность
The carcinogenicity of ethyleneimine was evaluated in two strains of mice, and both gave positive results. Groups of 18 male and 18 female mice of B6C3F1 or B6AKR strains were treated orally (initially by gavage, then in the diet) from age 7 days through 77–78 weeks. The time-weighted average (TWA) dose was about 1.8 mg/kg/day. The incidence of hepatomas and lung adenomas was significantly elevated in both strains and sexes. In B6C3F1 mice, the incidence of hepatomas and pulmonary adenomas was 15/17 and 15/17 in males and 11/15 and 15/15 females, respectively. In the B6AKR strain, hepatomas and adenomas occurred in 9/16 and 12/16 males and in 2/11 and 10/11 females, respectively. In the control groups, hepatomas were 8/79 and 0/87 in male and female B6C3F1 mice and 5/90 and 1/82 in male and female B6AKR mice. The respective incidence of pulmonary adenomas was 5/79, 3/87, 10/90, and 3/82. The incidence of hepatomas and pulmonary adenomas (reported as combined tumors) was significantly (p<0.01) elevated.Экологическая судьба
Photolytic. The vacuum UV photolysis (λ = 147 nm) and γ radiolysis of ethylenimine resulted in the formation of acetylene, methane, ethane, ethylene, hydrogen cyanide, methyl radicals, and hydrogen (Scala and Salomon, 1976). Photolysis of ethylenimine vapor at krypton and xenon lines yielded ethylene, ethane, methane, acetylene, propane, butane, hydrogen, ammonia, and ethyleneimino radicals (Iwasaki et al., 1973).Chemical/Physical. Polymerizes easily (Windholz et al., 1983). Hydrolyzes in water forming ethanolamine (HSDB, 1989). The estimated hydrolysis half-life in water at 25 °C and pH 7 is 154 d (Mabey and Mill, 1978).
Метаболизм
When male Dow-Wistar rats were injected intraperitoneally with [14C]-ethylenimine (80mug), approximately half of the dose was excreted in the urine (Wright and Rowe 1967). The major portion of the radioactivity in the urine consisted of unidentified products, although a small amount was excreted unchanged. A small portion, 3-5%, was expired as 14C02, and 1-3% was expired as a volatile, basic material, probably ethylenimine, during 24 h. Significant amounts of radioactivity were accumulated in liver, intestines, cecum, spleen, and kidneys. After 24 h, tissue radioactivity became constant and essentially unavailable for further metabolism. The aziridine ring of drugs is readily cleaved by microsomal enzymes, possibly with intermediate formation of an N-oxide (Oelschlager and Al Shaik 1985).Перевозки
UN1185 Ethyleneimine, stabilized, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Inhalation Hazard Zone A. PGIМетоды очистки
Redistil it in an Ar or N2 atmosphere in a fume hood, and store it over KOH in sealed bottles in a refrigerator. Commercial aziridine has been dried over sodium and distilled from the metal through an efficient column before use [Jackson & Edwards J Am Chem Soc 83 355 1961, Wenker J Am Chem Soc 57 2328 1935]. It is a weaker base than Me2NH (pK2 5 10.87) but is caustic to the skin. It should not be inhaled, causes inflammation of the eyes, nose and throat, and one may become sensitized to it. It is soluble in H2O, has an ammoniacal smell and reacts with CO2. Pure aziridine is comparatively stable but polymerises in the presence of traces of H2O and is occasionally explosive in the presence of acids. CO2 is sufficiently acidic to cause polymerisation (forms linear polymers) which is not free radical promoted. It is stable in the presence of bases. The violet 2:1 Cu complex crystallises from EtOH containing a few drops of aziridine and adding Et2O, and has m 142o(dec). The picrate has m 142o. [O'Rourke et al. J Am Chem Soc 78 2159 1956.] It has also been dried over BaO and has been distilled from sodium under nitrogen. [Allen et al. Org Synth Coll Vol IV 433 1963, Beilstein 20 III/IV 1.] TOXIC.Несовместимости
May form explosive mixture with air. Ethyleneimine is a medium strong base. Contact with acids, aqueous acid conditions, oxidizers, aluminum, or carbon dioxide may cause explosive polymerization. Explosive silver derivatives may be formed with silver alloys e.g., silver solder). Self-reactive with heat or atmospheric carbon dioxide. May accumulate static electrical charges, and may cause ignition of its vapors. Attacks rubber, coatings, plastics, and chemically active metals. Ethyleneimine vapors are not inhibited and may form polymers in vents or flame arresters, resulting in stopping of the vents.Утилизация отходов
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissionsМеры предосторожности
During use of ethyleninime, students and occupational workers should wear protective equipment, such as gloves, safety glasses, and should have good ventilation. Ethyleninime should be handled as a carcinogen. Ethyleninime vapor/air mixtures are explosive and pose a risk of fi re and explosion on contact with acid(s), oxidants.ЭТИЛЕНИМИН запасные части и сырье
сырьё
1of2
запасной предмет
- 1-АЗИРИДИНЭТАНОЛ
- Кармустин
- Triethylenethiophosphoramide
- ETHYL 2-CHLOROETHYLCARBAMATE
- 2-аминоэтантиол
- 2,2-диметилазиридин
- 2,5-BIS(1-AZIRIDINYL)-3,6-DICHLORO-2,5-CYCLOHEXADIENE-1,4-DIONE
- Цитиколин
- carboquone
- Полиэтиленимин
1of4