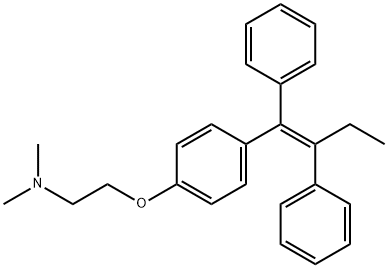
Тамоксифен
- английское имяTamoxifen
- CAS №10540-29-1
- CBNumberCB9438781
- ФормулаC26H29NO
- мольный вес371.51
- EINECS234-118-0
- номер MDLMFCD00010454
- файл Mol10540-29-1.mol
| Температура плавления | 97-98 °C(lit.) |
| Температура кипения | 501.18°C (rough estimate) |
| плотность | 1.0630 (rough estimate) |
| давление пара | 0Pa at 25℃ |
| показатель преломления | 1.6000 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: insoluble <0.1% at 20°C |
| пка | pKa 8.71(H2O t = 25 I = 0.025) (Uncertain) |
| форма | Solid |
| цвет | Crystals from pet ether |
| Растворимость в воде | Insoluble in water. Soluble in methanol, ethanol, propanol or propylene glycol.Soluble in dimethyl sulfoxide, dichloromethane and ethanol. Insoluble in water. |
| Мерк | 13,9137 |
| Стабильность | Light Sensitive |
| ИнЧИКей | NKANXQFJJICGDU-QPLCGJKRSA-N |
| LogP | 6.3 at 20℃ |
| Справочник по базе данных CAS | 10540-29-1(CAS DataBase Reference) |
| FDA UNII | 094ZI81Y45 |
| Код УВД | L02BA01 |
| МАИР | 1 (Vol. 66, 100A) 2012 |
| Система регистрации веществ EPA | Tamoxifen (10540-29-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.76 |
| Коды опасности | T,Xi | |||||||||
| Заявления о рисках | 45-60-61-64-36/37/38 | |||||||||
| Заявления о безопасности | 53-45-36-26 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KR5919600 | |||||||||
| кода HS | 29221990 | |||||||||
| Банк данных об опасных веществах | 10540-29-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orl-rat: 4100 mg/kg DRFUD4 9,186,84 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Тамоксифен химические свойства, назначение, производство
Описание
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized tamoxifen in the hope of developing a morning-after contraceptive pill. The UK patent for this compound was in place in 1962, whereas the US patent was repeatedly denied until the 1980s. Tamoxifen was approved for a fertility treatment but it was not proven as useful in regulating human contraception. Even though there was a link between estrogen and breast cancer, developing a cancer treatment was not a priority at the time. In 1971, the first clinical study showed a convincing effect of tamoxifen in treating advanced breast cancer. From 1971 to 1977, this drug was neither clinically nor financially remarkable. In 1980s, however, publications first showed that tamoxifen, in addition to chemotherapy, improved survival for patients with early stage breast cancer. In 1998, the meta-analysis by the Oxford-based Early Breast Cancer Trialists’ Collaborative Group showed that tamoxifen did indeed save lives in early breast cancer. In 2001, tamoxifen sales were over $1.024 billion. Since the expiration of the patent in 2002, it is now widely available as a generic drug. By 2004, tamoxifen was the best selling hormonal drug for the treatment of breast cancer.Химические свойства
White Crystalline SolidИспользование
A nonsteroidal estrogen antagonist of interest in the treatment of some forms of breast cancer. Tamoxifen is a Protein Kinase C inhibitor, and induces apoptosis in human malignant glioma cell linesПоказания
Tamoxifen (Nolvadex) is a synthetic antiestrogen used in the treatment of breast cancer. Normally, estrogens act by binding to a cytoplasmic protein receptor, and the resulting hormone–receptor complex is then translocated into the nucleus, where it induces the synthesis of ribosomal RNA (rRNA) and messenger RNA (mRNA) at specific sites on the DNA of the target cell. Tamoxifen also avidly binds to estrogen receptors and competes with endogenous estrogens for these critical sites. The drug–receptor complex has little or no estrogen agonist activity.Tamoxifen directly inhibits growth of human breast cancer cells that contain estrogen receptors but has little effect on cells without such receptors.Всемирная организация здравоохранения(ВОЗ)
Tamoxifen is an anti-estrogen agent used mainly to treat breast cancer. Tamoxifen is listed in the WHO Model List of Essential Drugs.Общее описание
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4. In breast cancer, the gene repressor activity of tamoxifen against ERBB2 is dependent upon PAX2. Blocks estradiol-stimulated VEGF production in breast tumor cells.Механизм действия
Tamoxifen is slowly absorbed, and maximum serum levels are achieved 4 to 7 hours after oral administration. The drug is concentrated in estrogen target tissues, such as the ovaries, uterus, vaginal epithelium, and breasts. Hydroxylation and glucuronidation of the aromatic rings are the major pathways of metabolism; excretion occurs primarily in the feces.Фармакокине?тика
Circulating levels of the demethylated metabolite at steady state are up to twice the level of the parent drug, because the elimination half-life of N-demethyl tamoxifen is 14 days, compared with 7 days for tamoxifen. Tamoxifen demonstrates only weak estrogenic effects at several sites, including the endometrium and bone, and on the lipid profile. Tamoxifen undergoes rapid N-dem ethylation to its major metabolite, N-dem ethyltamoxifen, by CYP3A4 and via CYP2D6 to its minor metabolite, 4-hydroxytam oxifen. Evidence suggests that 4-hydroxytamoxifen is the active metabolite of tamoxifen, with a higher binding affinity than the parent drug for the ERКлиническое использование
Tamoxifen is a SERM that is used as an antiestrogen in the treatment of estrogen-dependent breas Tcancer following prim ary treatment (c hemotherapy and/or surgery).Побочные эффекты
Tamoxifen administration is associated with few toxic side effects, most frequently hot flashes (in 10–20% of patients) and occasionally vaginal dryness or discharge. Mild nausea, exacerbation of bone pain, and hypercalcemia may occur.Профиль безопасности
Confirmed human carcinogen. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by an unspecified route: nausea or vomiting, leukopenia, thrombocytopenia, and skin changes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Канцерогенность
Tamoxifen is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.Тамоксифен запасные части и сырье
Тамоксифен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613315996897 | China | 792 | 58 | ||
| +86-13176845580 +86-13176845580 |
China | 232 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-13129979210 +86-13129979210 |
China | 376 | 58 | ||
| +8619956560829 | China | 286 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +8619031013551 | China | 144 | 58 | ||
| +undefined17712220823 | China | 615 | 58 | ||
| +8618873369619 | China | 260 | 58 |