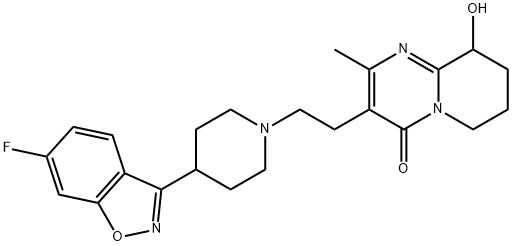
Палиперидон
- английское имяPaliperidone
- CAS №144598-75-4
- CBNumberCB9222359
- ФормулаC23H27FN4O3
- мольный вес426.48
- EINECS620-493-1
- номер MDLMFCD00871802
- файл Mol144598-75-4.mol
химическое свойство
| Температура плавления | 158-160°C |
| Температура кипения | 612.3±65.0 °C(Predicted) |
| плотность | 1.45±0.1 g/cm3(Predicted) |
| Fp | 9℃ |
| температура хранения | 2-8°C |
| растворимость | DMSO: soluble2mg/mL, clear (warmed) |
| пка | 13.00±0.60(Predicted) |
| форма | powder |
| цвет | white to brown |
| Стабильность | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| InChI | InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 |
| ИнЧИКей | PMXMIIMHBWHSKN-UHFFFAOYSA-N |
| SMILES | C12C(O)CCCN1C(=O)C(CCN1CCC(C3C4=C(ON=3)C=C(F)C=C4)CC1)=C(C)N=2 |
| Справочник по базе данных CAS | 144598-75-4(CAS DataBase Reference) |
| FDA UNII | 838F01T721 |
| Код УВД | N05AX13 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | T,F | |||||||||
| Заявления о рисках | 25-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 45-36/37-16 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UV1164720 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 144598-75-4(Hazardous Substances Data) | |||||||||
| Токсичность | rat,LD50,oral,65mg/kg (65mg/kg),Drug Development Research. Vol. 33, Pg. 399, 1994. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
-
оператор предупредительных мер
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
Палиперидон химические свойства, назначение, производство
Описание
Paliperidone, the C-9 hydroxylated active metabolite of the antipsychotic agent risperidone, is the newest atypical antipsychotic to join the market following the introductions of olanzapine (ZyprexaTM), risperidone (RisperdalTM), quetiapine(SeroquelTM), and ziprasidone (GeodonTM). Compared to its parent, paliperidone has improved PK properties and a reduced potential for drug interactions. In terms of receptor affinity, the two drugs are equipotent.Химические свойства
Off White to Light Orange Coloured SolidИспользование
Paliperidone(Invega) is an atypical antipsychotic. Chemically, paliperidone is the primary active metabolite of the older atypical antipsychotic risperidone. It is indicated for the acute and maintenance treatment of schizophreniaОбщее описание
Paliperidone, (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one(Invega), is essentially insoluble in water and is available asextended-release tablets. Paliperidone is delivered at a constantrate using an osmotic drug release device (OsmoticRelease Oral Systems [OROS]). The absolute bioavailabilityof paliperidone is 28%, and studies in healthy subjects on ahigh-fat, high-calorie meal showed an increase in AUC.Paliperidone is 74% bound to plasma proteins. After a single,1-mg dose of C-paliperidone, 59% of the dose was excretedin the urine as unchanged drug, and 32% of the dose was recoveredas metabolites. Most of the drug (80%) is excreted bythe kidneys. Paliperidone is metabolized by dealkylation, hydroxylation,dehydrogenation, and scission of the benzoxazolering. None of these metabolic pathways account for morethan 10% of the dose. The terminal elimination half-life ofpaliperidone is 23 hours.Побочные эффекты
The most common adverse events included tachycardia, QTc prolongation, headache, anxiety, dizziness, tremors, and insomnia along with the dose-related events of somnolence, orthostatic hypotension, salivary hypersecretion, and extrapyrimidal disorder. Weight gain was also observed in 6–9% of patients which may be attributable to paliperidone’s lower affinity for H1-histaminergic and a1- and a2-adrenergic receptors. Patients with renal impairment require dose adjustments since elimination of paliperidone is altered. Paliperidone is contraindicated in patients with a hypersensitivity to risperidone. Concomitant use of class III antiarrhythmic agents should be avoided since this may result in additive QT interval prolongation. Also, loss of levodopa efficacy is expected with this D2 antagonist.использованная литература
1) Beijsterveldt?et al.?(1994),?Regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat; Psychopharmacology,?114?53 DOI:10.1007/BF022454442) Leysen?et al. (1994),?Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity; J. Clin. Psychiatry,?55?suppl: 5 PMID: 7520908
3) Schotte?et al.?(1996),?Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding; Psychopharmacology (Berl.),?124?57 DOI:10.1007/BF02245606
Палиперидон запасные части и сырье
Палиперидон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15552509998 +86-15621883869 |
China | 251 | 58 | ||
| +1-817863-6948 +1-(817)863-6948 |
United States | 19632 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| 025-83697070 | CHINA | 3009 | 60 |