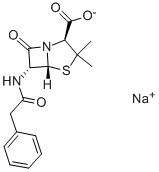
Пенициллин G натриевая сол
- английское имяPenicillin G sodium salt
- CAS №69-57-8
- CBNumberCB9211940
- ФормулаC16H17N2NaO4S
- мольный вес356.37
- EINECS200-710-2
- номер MDLMFCD00069666
- файл Mol69-57-8.mol
| Температура плавления | 209-212°C |
| альфа | D24.8 +301° (c = 2.0 in water) |
| плотность | 1.41 |
| показатель преломления | 300 ° (C=2, H2O) |
| температура хранения | Inert atmosphere,2-8°C |
| растворимость | H2O: 100 mg/mL Solutions should be filter sterilized and stored at 2-8°C for 1 week or at -20°C for extended periods. Solutions are stable at 37°C for 3 days. |
| форма | powder |
| цвет | colorless or white |
| РН | 5.5-7.5 (3% in H2O) |
| Растворимость в воде | 5-10 g/100 mL at 25 ºC |
| Мерк | 14,7094 |
| БРН | 3834217 |
| Стабильность | Stability Stable, but incompatible with a wide variety of materials, including acids, oxidizing agents, heavy metals, alcohols, glycerol, thiomersal, many surface-active agents, alkalies, peroxides, reducing agents, lanolin, glycol, sugars, amines, iodine, iodides, thiols. Hygroscopic. |
| Справочник по базе данных CAS | 69-57-8(CAS DataBase Reference) |
| FDA 21 CFR | 556.510 |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | penicillin |
| FDA UNII | YS5LY7JF4N |
| Словарь наркотиков NCI | picibanil |
| Система регистрации веществ EPA | Penicillin G sodium (69-57-8) |
| UNSPSC Code | 12352207 |
| NACRES | NA.76 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 42/43 | |||||||||
| Заявления о безопасности | 22-36/37-45 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | XH9800000 | |||||||||
| F | 10-23 | |||||||||
| кода HS | 29411000 | |||||||||
| Токсичность | LD50 oral in rat: 6916mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
Пенициллин G натриевая сол химические свойства, назначение, производство
Химические свойства
White powderИстория
Penicillin was discovered by chance in 1928 by Alexander Fleming. He observed that the growth of a bacteria culture was inhibited by a fungus Penicillum notatum. He published his results but did not pursue its industrial development actively. Ten years later, H. Florey and coworkers had produced enough purified penicillin to treat just one patient. This test, however, was sufficient to prove that it was a viable drug. From then on many people and companies participated in the development of new fermentation technologies, new microorganisms, new downstream processing, and so on to make a large-scale production possible. Penicillin did not only change the medical world, but also the fermentation technology. The naturally growing (wild type) Penicillum notatum produced penicillin with a yield of 10 mg/L. Therefore, the first task was the search for a more productive species. Eventually, Penicillium chrysogenum was identified as the most productive species. To enhance penicillin production further, the old method of growing Penicillum mold on the surface of the medium in liter-sized flasks was replaced by fermentation in large aerated tanks. This allowed the mold to grow throughout the entire tank and not just on the surface of the medium. Today, penicillin and other antibiotics are produced in large-scale fermenters holding several hundred cubic meters of medium and the yield has increased 5000 fold to 50 g/L.Использование
Penicillin G sodium salt is used as a β-lactam antibiotic. Penicillin G can be used as a selective agent in several types of isolation media. It has been used to study the diagnostic and therapeutic implications of gentamicin-resistant Enterococcus faecalis sequence type 6 with reduced penicillin susceptibility and in cell culture both alone and combined with streptomycin and other antibiotics.Определение
penicillin: An antibiotic derivedfrom the mould Penicillium notatum;specifically it is known as penicillin Gand belongs to a class of similar substancescalled penicillins. They producetheir effects by disruptingsynthesis of the bacterial cell wall,and are used to treat a variety of infectionscaused by bacteria.Всемирная организация здравоохранения(ВОЗ)
Penicillin is listed separately in the WHO Model List of Essential Drugs.Общее описание
White to slightly yellow crystalline powder with a faint odor. pH (10% solution) 5.5-7.5.Реакции воздуха и воды
Water soluble.Профиль реактивности
Penicillin G sodium salt is hygroscopic. Penicillin G sodium salt is incompatible with acids, oxidizing agents (especially in the presence of trace metals), heavy metal ions such as copper, lead, zinc and mercury; glycerol, sympathomimetic amines, thiomersal, wood alcohols, cetostearyl alcohol, hard paraffins, macrogols, cocoa butter and many ionic an nonionic surface-active agents. Penicillin G sodium salt is also incompatible with alkalis, compounds leached from vulcanized rubber, hydrochlorides of tetracyclines and organic peroxides. Other incompatibilities include reducing agents, alcohols, other hydroxy compounds, self-emulsifying stearyl alcohol, emulsifying wax, lanolin, crude cholinesterated bases, glycol, sugars, amines, aminacrine hydrochloride, ephedrine, procaine, rubber tubing, thiamine hydrochloride, zinc oxide, oxidized cellulose, iodine, iodides, thiols, chlorocresol and resorcinol. Penicillin G sodium salt may also be incompatible with naphthalene oils and vitamin B.Пожароопасность
Flash point data for Penicillin G sodium salt are not available; however, Penicillin G sodium salt is probably combustible.Профиль безопасности
Poison by intracerebral,parenteral, and intramuscular routes. Moderately toxic viaintravenous route. Mildly toxic by ingestion. Experimentalteratogenic and reproductive effects. Questionablecarcinogen with experimental tumorigenic data. Whenheated toМетоды очистки
Purify the salt by dissolving it in a small volume of MeOH (in which it is more soluble than EtOH) and treating gradually with ~5 volumes of EtOAc. This gives an almost colourless crystalline solid (rosettes of clear-cut needles) and recrystallising twice more if slightly yellow in colour. The salt has also been conveniently recrystallised from the minimum volume of 90% Me2CO and adding an excess of absolute Me2CO. A similar procedure can be used with wet n-BuOH. If yellow in colour, then dissolve (~3.8g) in the minimum volume of H2O (3mL), add n-BuOH and filter through a bed of charcoal. The salt forms long white needles on standing in a refrigerator overnight. More crystals can be obtained on concentrating the mother liquors in vacuo at 40o. A further recrystallisation (without charcoal) yields practically pure salt. A good preparation has ~600 Units/mg. The presence of H2O in the solvents increases the solubility considerably. The solubility in mg/100mL at 0o is 6.0 (Me2CO), 15.0 (Me2CO/0.5% H2O), 31.0 (Me2CO/1.0% H2O), 2.4 (methyl ethyl ketone), 81.0 (n-butanol) and 15.0 (dioxane at 14o). Alternatively it is dissolved in H2O (solubility is ~10%), filtered if necessary and precipitated by addition of EtOH and dried in a vacuum over P2O5. A sample can be kept for 24hours at 100o without loss of physiological activity. It also crystallises from MeOH/EtOAc. [IR: Barnes et al. Anal Chem 19 620 1947, The Chemistry of Penicillin (Clarke, Johnson and Robinson eds.) Princeton University Press, Princeton NJ, Chapter V 85 1949, Beilstein 27 III/IV 5861.]Пенициллин G натриевая сол запасные части и сырье
Пенициллин G натриевая сол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-66697723 +86-17703311139 |
China | 563 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8615102730682 | CHINA | 566 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |