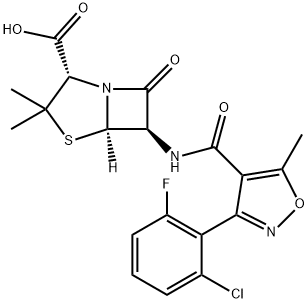
Флуклоксациллин
- английское имяFlucloxacillin
- CAS №5250-39-5
- CBNumberCB8690991
- ФормулаC19H17ClFN3O5S
- мольный вес453.87
- EINECS226-051-0
- номер MDLMFCD00864886
- файл Mol5250-39-5.mol
химическое свойство
| Температура кипения | 677.3±55.0 °C(Predicted) |
| плотность | 1.59±0.1 g/cm3(Predicted) |
| растворимость | soluble in Methanol, Water |
| пка | pKa 2.7 (Uncertain) |
| форма | Solid |
| цвет | White |
| Справочник по базе данных CAS | 5250-39-5(CAS DataBase Reference) |
| FDA UNII | 43B2M34G2V |
| Код УВД | J01CF05 |
Флуклоксациллин химические свойства, назначение, производство
Описание
Chemically this is 3(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl penicillin; this differs from dicloxacillin only by the substitution of a fluorine for a chlorine atom (Sutherland et al., 1970). It comes as oral capsules of 250 and 500 mg, as a suspension of 25 and 50 mg/ml, and in an injectable formulation of 500 mg and 1 g.Использование
Antibacterial.Определение
ChEBI: A penicillin compound having a 6beta-[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-carboxamido] side-chain.Антимикробная активность
There is complete cross-resistance with other penicillinase-stable penicillins.Общее описание
Flucloxacillin was synthesized by Beecham Research Laboratories in 1962 as a penicillinase-stable and orally active semisynthetic penicillin. It shows almost the same activity as dicloxacillin, and it has slightly higher serum and tissue concentrations than dicloxacillin. This drug has been used to treat pyoderma, sepsis, and postoperative infections as well as ear and nose, respiratory tract, and other infections caused by Staphylococcus and Streptococcus, including benzylpenicillin-resistant strains.Фармакокине?тика
Oral absorption: c. 80%Cmax 250 mg (oral): 11 mg/L after 0.5–1 h
Plasma half-life: 2 h
Plasma protein binding: 95%
Absorption and distribution
It is well absorbed after oral administration and penetrates rapidly into extravascular exudates. Its high protein binding limits its diffusion, notably into the normal CSF.
Metabolism and excretion
Flucloxacillin is partly metabolized in the liver and about 10% of the plasma concentration is made up of metabolites. It is more slowly eliminated than cloxacillin. Some appears in the bile but about 50–80% of an oral dose is recovered from the urine, about 20% as metabolites.
Клиническое использование
Uses are those of group 3 penicillins.Побочные эффекты
In patients treated by intravenous infusion, about 5% developed phlebitis by the first and 15% by the second day, after which the proportion rose dramatically. Side effects are otherwise those common to penicillins.Флуклоксациллин запасные части и сырье
Флуклоксациллин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +8618058761490 | China | 49983 | 58 |