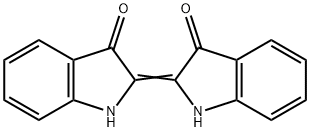
Индиго
- английское имяIndigo
- CAS №482-89-3
- CBNumberCB7459700
- ФормулаC16H10N2O2
- мольный вес262.26
- EINECS207-586-9
- номер MDLMFCD00005722
- файл Mol482-89-3.mol
| Температура плавления | >300 °C(lit.) |
| Температура кипения | 405.51°C (rough estimate) |
| плотность | 1.01 g/mL at 20 °C |
| давление пара | 0Pa at 100℃ |
| показатель преломления | 1.5800 (estimate) |
| Fp | >220℃ |
| температура хранения | Sealed in dry,Room Temperature |
| растворимость | DMSO (Slightly, Heated, Sonicated), DMF (Slightly) |
| форма | Powder |
| Цветовой индекс | 73000 |
| пка | -3.83±0.20(Predicted) |
| цвет | Dark blue to violet |
| Растворимость в воде | <0.1 g/100 mL |
| λмакс | 602 nm |
| ε(коэффициент экстинкции) | ≥8000 at 599-605nm in chloroform |
| Мерк | 14,4943 |
| БРН | 88275 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | COHYTHOBJLSHDF-BUHFOSPRSA-N |
| LogP | 2.7 at 23℃ |
| FDA 21 CFR | 74.3106 |
| Справочник по базе данных CAS | 482-89-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | 1G5BK41P4F |
| Справочник по химии NIST | c.i. Vat blue 1(482-89-3) |
| Система регистрации веществ EPA | C.I. Vat Blue 1 (482-89-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.47 |
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/38-36/37/38-48/20/21/22 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| РИДАДР | UN 3264 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DU2988400 | |||||||||
| кода HS | 32041510 | |||||||||
| Банк данных об опасных веществах | 482-89-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in mouse: > 32gm/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Индиго химические свойства, назначение, производство
Описание
Indigo, known chemically as indigotin, is a common blue dye that has been highly valued throughout history and has played a major role in trade and commerce since ancient times. The term indigo is often used to describe many blue dyes produced from a number of plants. For example, woad, a blue dye obtained from the plant Isatis tinctoria, was used throughout the Mediterannean and Europe and is often identified as indigo. True indigo comes from the leguminous plant of the genus Indigofera.The Indigofera genus includes several hundred species, and indigo has been obtained from a number of these, but the dominant species for the dye are Indigofera tinctoria grown mainly in India and tropical Asia and Indigofera suff ructiosa from the tropical Americas. The name indigo comes from the Greek indikon and Latin indicum meaning “dye from India.” There is evidence that indigo was used several thou sand years b.c.e. Persian rugs containing indigo color exist from several thousand years b.c.e. Textile artifacts from Egyptian tombs provide evidence of indigo’s use by royalty from as far back as 2500 b.c.e. The writings of Herodotus from approximately 450 b.c.e. mention indigo’s use in the Mediterranean area.
Химические свойства
dark violet powderВхождение
Indigo is a perennial shrub found in several regions of the world.История
Indigotin. The blue dye of the ancient world was derived from indigo and woad. Which plant is the oldest is a matter of conjecture. That indigo was known at least four thousand years ago is evident from ancient Sanskrit writings. Cloth dyed with indigotin (CI Natural Blue; CI 75780) has been found in Egyptian tombs and in the graves of the Incas in South America. Indigo belongs to the legume family. The two most important species are Indigo tinctoria and I. suffruticosa, found in India and the Americas, respectively. The leaves of the indigo plant do not contain the dye as such, but in the form of its precursor, a glycoside known as indican.Использование
In recent years researchers have used genetic engineering using Escherichia coli to convert tryptophan into indigo. The desire for natural organic products has also revived traditional production methods of indigo on a small scale. Indigo's dominant use is as a textile dye, but indigo-related compounds have limited use as indicators and in food coloring.the Food and Drug Administration's FD&C Blue #2 contains indigotine (also known as indigo carmine), which is a sulfonated sodium salt of indigo.Определение
indigo: A blue vat dye, C16H10N2O2.It occurs as the glucoside indican inthe leaves of plants of the genus Indigofera,from which it was formerlyextracted. It is now made synthetically.Методы производства
The first synthesis of indigo is attributed to Adolf von Baeyer (1835–1917), who began hisquest to synthesize indigo in 1865 but was not able to produce indigo until 1878. The syntheticproduction of indigo was first described by Baeyer and Viggo Drewson in 1882; Baeyeralso identified the structure of indigo in 1882.the Baeyer-Drewson synthesis of indigo startedwith 2-nitrobenzaldehyde and acetone proceeding through a series of steps in alkali solution.Baeyer’s work was not commercially viable, and it was not until 1897 that BASF (BadischeAnalin und Soda Fabrik) started to produce indigo commercially using a process developedby Karl von Heumann (1851–1894) that started with naphthalene. The synthetic productionof indigo spelled the end of traditional methods of indigo production. By the second decadeof the 20th century, nearly all indigo was produced synthetically.Биологические функции
Indigo naturalis has a variety of pharmacological activities, such as anti-inflammatory, antioxidant, antibacterial, antiviral, immunomodulatory and so on. It has very good clinical effect on psoriasis, leukemia and ulcerative colitis.Общее описание
Dark blue powder with coppery luster. Occurs in isomeric forms (cis and trans). In solid state Indigo is in the trans form.Реакции воздуха и воды
Insoluble in water.Угроза здоровью
ACUTE/CHRONIC HAZARDS: Indigo may cause irritation of the skin and mucous membranes.Пожароопасность
Flash point data for Indigo are not available but Indigo is probably combustible.Профиль безопасности
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx.Методы очистки
First reduce indigo in alkaline solution with sodium hydrosulfite, and filter. The filtrate is then oxidised by air, and the resulting precipitate is filtered off, dried at 65-70o, ground to a fine powder, and extracted with CHCl3 in a Soxhlet extractor. Evaporation of the CHCl3 extract gives the purified dye. [Brode et al. J Am Chem Soc 76 1034 1954; spectral characteristics are listed, Beilstein 24 II 233, 24 III/IV 1791.]Индиго запасные части и сырье
сырьё
1of4
запасной предмет
1of2
Индиго поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8613815430202 | CHINA | 376 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 |