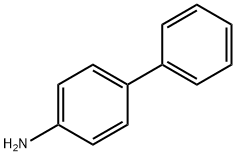
4-АМИНОБИФЕНИЛ
- английское имя4-Aminobiphenyl
- CAS №92-67-1
- CBNumberCB7229899
- ФормулаC12H11N
- мольный вес169.22
- EINECS202-177-1
- номер MDLMFCD00007879
- файл Mol92-67-1.mol
| Температура плавления | 52-54 °C(lit.) |
| Температура кипения | 191 °C15 mm Hg(lit.) |
| плотность | 1.1154 (rough estimate) |
| давление пара | 6 x 10-5 mmHg at 20–30 °C (quoted, Mercer et al., 1990) |
| показатель преломления | 1.5785 (estimate) |
| Fp | >110°C |
| температура хранения | -20°C Freezer |
| растворимость | Soluble in Dichloromethane, DMSO and Methanol |
| пка | 4.35(at 18℃) |
| форма | powder |
| цвет | Brown to Dark Yellow |
| Растворимость в воде | 842 mg/L at 20–30 °C (quoted, Mercer et al., 1990) |
| Мерк | 13,1235 |
| БРН | 386533 |
| констант закона Генри | (x 1010 atm?m3/mol): 3.89 at 25 °C (calculated, Mercer et al., 1990) |
| Стабильность | Stable, but slowly reacts with oxygen in the air. Combustible. Incompatible with strong oxidizing agents, acids, acid anhydrides, acid chlorides. |
| ИнЧИКей | DMVOXQPQNTYEKQ-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 92-67-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6 |
| FDA UNII | 16054949HJ |
| Предложение 65 Список | 4-Aminobiphenyl (4-aminodiphenyl) |
| МАИР | 1 (Vol. 1, Sup 7, 99, 100F) 2012 |
| Справочник по химии NIST | 4-Aminobiphenyl(92-67-1) |
| Система регистрации веществ EPA | 4-Aminobiphenyl (92-67-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,Xn | |||||||||
| Заявления о рисках | 45-22-36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 53-45-36-26 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | DU8925000 | |||||||||
| Температура самовоспламенения | 842 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29214990 | |||||||||
| Банк данных об опасных веществах | 92-67-1(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 200 mg/kg, mice 50 mg/kg (quoted, Verschueren, 1983). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H350:Может вызывать раковые заболевания.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
4-АМИНОБИФЕНИЛ химические свойства, назначение, производство
Описание
4-Aminobiphenyl is an aromatic amine (arylamine) that exists at room temperature as a colorless crystalline solid with a floral odor. It is slightly soluble in cold water, but readily soluble in hot water. It is soluble in ethanol, ether, acetone, chloroform, and lipids. It oxidizes in air and emits toxic fumes when heated to decomposition (Akron 2009).Физические свойства
Colorless to yellow-brown crystalline solid with a floral-like odor. Becomes purple on exposure to air.Использование
In the United States, 4-aminobiphenyl now is used only in laboratory research. It formerly was used commercially as a rubber antioxidant, as a dye intermediate, and in the detection of sulfates (HSDB 2009).Определение
ChEBI: 4-Aminobiphenyl is an aminobiphenyl that is biphenyl substituted by an amino group at position 4. It has a role as a carcinogenic agent. It derives from a hydride of a biphenyl.Общее описание
Colorless to yellowish-brown crystals or light brown solid.Реакции воздуха и воды
Is oxidized by air (darkens on oxidation). Insoluble in water.Профиль реактивности
4-AMINOBIPHENYL is a weak base. Incompatible with acids and acid anhydrides. Forms salts with hydrochloric acid and sulfuric acid. Can be diazotized, acetylated and alkylated. . May react with strong oxidizing agents.Опасность
Toxic by ingestion, inhalation, skin absorp- tion. Confirmed carcinogen. Bladder and liver can- cer.Угроза здоровью
4-Aminodiphenyl exposure is associated with a high incidence of bladder cancer in humans.Пожароопасность
4-AMINOBIPHENYL is probably combustible.Профиль безопасности
Confirmed human carcinogen with experimental carcinogenic and tumorigenic data. Poison by ingestion and intraperitoneal routes. Human mutation data reported. An irritant. Effects resemble those of benzidine. See also BENZIDINE. Slight to moderate fire hazard when exposed to heat, flames (sparks), or powerful oxidzers. To fight fire, use water spray, mist, dry chemical. When heated to decomposition it emits toxic fumes of NOx,. See also AROMATIC AMINES.Возможный контакт
4-Aminobiphenyl is no longer manufactured commercially and is only used for research purposes. 4-Aminobiphenyl was formerly used as a rubber antioxidant and as a dye intermediate. Is a contaminant in 2-aminobiphenyl.Канцерогенность
4-Aminobiphenyl is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.Cancer of the urinary bladder was first reported to be associated with occupational exposure to 4-aminobiphenyl in a descriptive epidemiological study (published in the mid 1950s), in which 11% (19 of 171) of workers in a plant manufacturing 4-aminobiphenyl developed urinary-bladder cancer. These workers had been exposed to 4-aminobiphenyl for 1.5 to 19 years between 1935 and 1955. Publication of this study led to an effort to discontinue production and useof 4-aminobiphenyl. Starting in 1955, 541 workers who had been exposed to 4-aminobiphenyl were followed for an additional 14 years; 43 men (7.9%) developed histologically confirmed urinary-bladder cancer. In a survey of workers at a plant producing a variety of chemicals, the risk of mortality from urinary-bladder cancer was elevated tenfold, and all of the men who died of urinary-bladder cancer had worked at the plant during the period when 4-aminobiphenyl was used (1941 through 1952). The International Agency for Research onCancer concluded that there was sufficient evidence of the carcinogenicity of 4-aminobiphenyl in humans (IARC 1972, 1987).
Since 4-aminobiphenyl was listed in the First Annual Report on Carcinogens, most research on its carcinogenicity has focused on exposure from cigarette smoking. Epidemiological studies have reported the incidence of urinary-bladder cancer to be 2 to 10 times as high among cigarette smokers as among nonsmokers. Higher levels of 4-aminobiphenyl adducts (4-aminobiphenyl metabolites bound to DNA or protein) were detected in bladder tumors (DNA adducts) and red blood cells (hemoglobin adducts) from smokers thanfrom nonsmokers (Feng et al. 2002). In a case-control study, levels of 4-aminobiphenyl–hemoglobin adducts were higher in smokers with urinary-bladder cancer than in a control group of similarly exposed smokers (Del Santo et al. 1991). A Taiwanese study reported that 4-aminobiphenyl–hemoglobin adducts were associated with increased risk of liver cancer (Wang et al. 1998).
Экологическая судьба
4-Aminobiphenyl is one of a number of chemicals that cause methemoglobinemia, or conversion of hemoglobin to methemoglobin, which reduces the ability of the blood to carry oxygen to the tissues. In addition, the active metabolite is believed to produce cancer through its reaction with cellular DNA. In animal studies, the observed incidence of 4-aminobiphenyl adducts with bladder epithelium DNA correlated well with the observed bladder tumor incidence.Метаболический путь
Ring oxidation of 4-aminobiphenyl occurred only to a minor extent in microsomes. In contrast, N-oxidation of 4,4'-methylene-bis-(2-chloroaniline) is preferentially catalyzed by the phenobarbital-induced enzymes P- 450PB-B and P-450PB-D to cause ring oxidation and methylene carbon oxidation. 4,4'-Methylene-bis-(2- chloroaniline) ring oxidation and methylene carbon oxidation show varied cytochrome P-450 selectivity and accounted for 14-79% of total oxidation products.Перевозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name RequiredМетоды очистки
Crystallise it from water or EtOH. [Beilstein 12 IV 3241.] CARCINOGENIC.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides and acid anhydrides.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal devices.4-АМИНОБИФЕНИЛ запасные части и сырье
сырьё
1of2
запасной предмет
1of2
4-АМИНОБИФЕНИЛ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-512 65869404 +86-18934593683 |
China | 257 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +86-16632316109 | China | 1085 | 58 | ||
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 |