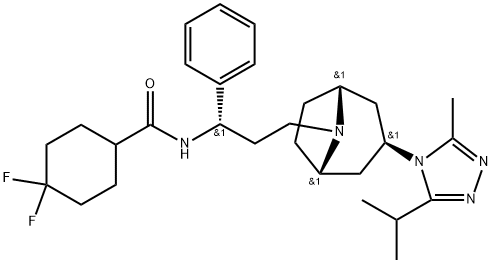
Маравирок
- английское имяMaraviroc
- CAS №376348-65-1
- CBNumberCB71270957
- ФормулаC29H41F2N5O
- мольный вес513.68
- EINECS609-456-0
- номер MDLMFCD09953791
- файл Mol376348-65-1.mol
химическое свойство
| Температура плавления | 79-81°C |
| плотность | 1.29±0.1 g/cm3(Predicted) |
| температура хранения | room temp |
| растворимость | DMSO: >30mg/mL |
| пка | 7.3(at 25℃) |
| форма | white powder |
| цвет | White |
| оптическая активность | [α]-15/D |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| ИнЧИКей | GSNHKUDZZFZSJB-RWJISDSDNA-N |
| SMILES | C(N1[C@@H]2CC[C@H]1C[C@H](N1C(=NN=C1C(C)C)C)C2)C[C@@H](C1C=CC=CC=1)NC(C1CCC(F)(F)CC1)=O |&1:2,5,7,19,r| |
| Справочник по базе данных CAS | 376348-65-1(CAS DataBase Reference) |
| FDA UNII | MD6P741W8A |
| Словарь наркотиков NCI | maraviroc |
| Код УВД | J05AX09 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
| Коды опасности | Xn |
| Заявления о рисках | 48/22 |
| Заявления о безопасности | 22-36 |
| WGK Германия | 1 |
| Банк данных об опасных веществах | 376348-65-1(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Маравирок химические свойства, назначение, производство
Описание
Maraviroc is the first CCR5 receptor antagonist that has been developed and launched for the treatment of HIV-1. Maraviroc binds in a slowly reversible, allosteric manner to CCR5, which is one of two principle chemokine co-receptors for viral entry into the host cell, the other being CXCR4. Binding of maraviroc to CCR5 induces conformational changes within the chemokine receptor, thereby preventing CCR5 binding to the viral gp120 protein and the ultimate CCR5- mediated virus-cell fusion that is a prerequisite for HIV invasion. Maraviroc, with its unique mechanism of action as a fusion inhibitor, joins the greater than 20 marketed antiretrovirals, including nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and PIs. It is approved for use in combination with these other antiretroviral drugs in adult patients with R5-tropic HIV-1 infection (but not X4 or dual/mixed tropic HIV-1).Химические свойства
Brown SolidИспользование
Maraviroc is a CCR5 antagonist for MIP-1α, MIP-1β and RANTES with IC50 of 3.3 nM, 7.2 nM and 5.2 nM, respectivelyОпределение
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 4,4-difluorocyclohexanecarboxylic acid and the primary amino group of (1S)-3-[(3-exo)-3-(3-isopropyl-5-methyl-4H-1,2,4- riazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropylamine. An antiretroviral drug, it prevents the interaction of HIV-1 gp120 and chemokine receptor 5 (CCR5) necessary for CCR5-tropic HIV-1 to enter cells.Приобретенная устойчивость
In most patients (c. 60%) failure of response is associated with the selection of virus that can use CXCR4 as its entry co-receptor. Evidence for the selection of virus that continues to use CCR5 has also been described.Фармацевтические приложения
A spirodiketopiperazine formulated as tablets for oral use.Фармакокине?тика
Oral absorption: c. 33% (300 mg dose)Cmax 150 mg twice daily: c. 332 μg/L*
Cmin 150 mg twice daily: c. 101 μg/L*
Plasma half-life: c. 13.2 h (30 mg iv administration)
Volume of distribution: c. 194 L
Plasma protein binding: c. 76%
Absorption
The absolute bioavailability of a 100 mg dose is 23% and is predicted to be 33% after a 300 mg dose. Co-administration of a 300 mg tablet and a high-fat meal has resulted in reduced Cmax and AUC by 33% in healthy volunteers. However, because no food restrictions were enacted during clinical trials, maraviroc may be taken with or without food.
Distribution
Animal experiments suggest low CSF concentrations around 10% of free plasma concentrations. It is not known whether it passes into breast milk. A study of genital tract secretions and vaginal tissue in healthy HIV-uninfected female volunteers suggest a concentration in cervicovaginal fluid more than four-fold higher than that in plasma.
Metabolism
It is a substrate for CYP3A4 and P-glycoprotein, but does not appear to inhibit or induce CYP3A4.
Excretion
Seventy-six and 19% of a radiolabeled maraviroc dose were recovered in the feces and urine, respectively.
Клиническое использование
Treatment of HIV infection (in combination with other antiretroviral drugs) in treatment-experienced patientsOn November 20, 2009, the US Food and Drug Administration approved a supplemental new drug application to expand the indication for maraviroc to include combination antiretroviral treatment of treatmentnaive adults infected with CCR5-tropic HIV virus
Побочные эффекты
Overall, maraviroc was well tolerated with the most common adverse events being cough, fever, colds, rash, muscle and joint pain, stomach pain, and dizziness. While some patients did experience liver enzyme elevation, these events did not appear to be doserelated. Since hepatotoxicity did occur in one patient with prior liver function abnormalities, maraviroc s label warns of a potentially increased risk of hepatoxicity with treatment. Postural hypotension was also observed in a dosedependent manner; however, no patients discontinued therapy as a result. As a substrate for CYP3A4, the dose of maraviroc should be reduced by 50% in the presence of strong CYP3A4 inhibitors. Conversely, concomitant use of strong CYP3A4 inducers requires a 50% increase in maraviroc dose. While there are no contraindications, maraviroc should be used with caution in patients with liver dysfunction, high risk of cardiovascular events, and pre-existing postural hypotension.Маравирок запасные части и сырье
сырьё
- N-Бензилтропинон
- (1S)-3-[3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-exo-8-azabicyclo[3.2.1]oct-8-yl]-1-phenyl-1-propanamine
- 4,4-Difluorocyclohexanecarboxylic кислота
- 4,4-DIFLUORO-N-((1S)-3-OXO-1-PHENYLPROPYL)CYCLOHEXANE-1-CARBOXAMIDE
- 8-Benzyl-3-exo-(5-isopropyl-3-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane
- METHYL (3S)-3-AMINO-3-PHENYLPROPANOATE
- (S)-трет-бутил-3-оксо-1-фенилпропилкарбамат
- (1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane
- ((S)-3-[3-(3-ISOPROPYL-5-METHYL-[1,2,4]TRIAZOL-4-YL)-8-AZA-BICYCLO[3.2.1]OCT-8-YL]-1-PHENYL-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER
- Циклогексанкарбонилхлорид, 4,4-дифтор-(9CI)
1of3
Маравирок поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618327326525 | China | 8467 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +8615387054039 | China | 427 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-17332992504 +86-17332992504 |
China | 300 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7782 | 55 |