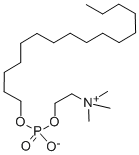
Милтефосин
- английское имяMiltefosine
- CAS №58066-85-6
- CBNumberCB6370752
- ФормулаC21H46NO4P
- мольный вес407.57
- EINECS622-572-6
- номер MDLMFCD00133396
- файл Mol58066-85-6.mol
химическое свойство
| Температура плавления | 232-234° (dec) |
| температура хранения | room temp |
| растворимость | H2O: soluble10mg/mL, clear, colorless |
| форма | Crystalline solid |
| цвет | White to Almost white |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | H2O: 10mg/mL, clear, colorless |
| InChI | InChI=1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3 |
| ИнЧИКей | PQLXHQMOHUQAKB-UHFFFAOYSA-N |
| SMILES | O(CCCCCCCCCCCCCCCC)P([O-])(=O)OCC[N+](C)(C)C |
| Справочник по базе данных CAS | 58066-85-6(CAS DataBase Reference) |
| FDA UNII | 53EY29W7EC |
| Словарь наркотиков NCI | miltefosine |
| Код УВД | P01CX04 |
| UNSPSC Code | 51191904 |
| NACRES | NA.85 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-43 | |||||||||
| Заявления о безопасности | 36/37 | |||||||||
| РИДАДР | UN 2811 6.1 / PGIII | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KH2890000 | |||||||||
| кода HS | 29239000 | |||||||||
| Токсичность | LD50 in rats (mg/kg): 246 orally (Muschiol) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Милтефосин химические свойства, назначение, производство
Описание
Miltefosin, representing the prototype of a new phospholipid structure, was introduced for the palliative treatment of skin metastases in patients with breast cancer. It is highly active against the human leukemia tumor cells xenograft in nude mice, leading to growth inhibition and regression of large established tumors. Its mode of antitumor activity is not mediated by the host immune system but by its pharmacological effects at the level of the cancer cell membrane, distinctly different from that of the classical cytostatic drugs which interact with cell proliferation at the level of DNA replication. Protein kinase C inhibition has been suggested as a possible mechanism.Использование
A phospholipid drug with antineoplastic and antiprotozoal/antifungal properties, also acts as an Akt inhibitor, and under investigation as a potential therapy against HIV infection.Определение
ChEBI: A phospholipid that is the hexadecyl monoester of phosphocholine.Антимикробная активность
Concentrations of 1–5 μm inhibit the promastigotes and amastigotes of Leishmania spp. and the epimastigotes and amastigotes of T. cruzi. Inhibitory concentrations against T. brucei spp. and E. histolytica are closer to 50 μm. Acanthamoeba spp. are variably susceptible, depending on the experimental conditions.Приобретенная устойчивость
There are no reports of clinical resistance in Leishmania so far. Experimental resistance has been induced in vitro against the promastigote stage of Leishmania and two plasma membrane proteins, LdMT and Ld Ros3, are necessary for miltefosine uptake. There is evidence that reduced sensitivity of promastigotes is passed on to intracellular amastigotes.Фармацевтические приложения
An alkylphospholipid, originally investigated as an anticancer compound, formulated for oral administration.Фармакокине?тика
In rodent models the drug is almost completely absorbed after oral administration. About 90% is bound to plasma proteins. It is widely distributed in the body; studies in rats showed highest uptake in kidney, liver and spleen. In rats and dogs bioavailability was 82% and 94%, with maximum values reached after 4–48 h.In adult human trials repeated oral dosing with 100 mg per day achieved a peak plasma concentration of 70 mg/L after 8–24 h (day 23). The half-life is 6–8 days.
Клиническое использование
Visceral leishmaniasisCutaneous leishmaniasis
Побочные эффекты
Mild to moderate gastrointestinal side effects are reported in 40–60% of patients. Moderate to severe nephrotoxicity was seen in 2% and 1% of patients, respectively; increases in creatinine levels were reversible. Miltefosine is contraindicated in pregnancy, based on findings of teratogenicity in rats. It causes hemolysis and cannot be given intravenously.Милтефосин запасные части и сырье
Милтефосин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613651027935 | China | 191 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 18953170293 | China | 2930 | 58 |