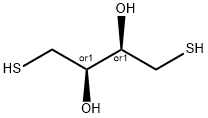
DL-Dithiothreitol
- русский язык имя
- английское имяDL-Dithiothreitol
- CAS №3483-12-3
- CBNumberCB6342919
- ФормулаC4H10O2S2
- мольный вес154.25
- EINECS222-468-7
- номер MDLMFCD00004877
- файл Mol3483-12-3.mol
| Температура плавления | 41-44 °C(lit.) |
| Температура кипения | 125 °C |
| альфа | -0.2~+0.2°(20℃/D)(c=5,H2O) |
| плотность | 1.04 g/mL at 20 °C |
| Плотность накопления | 300kg/m3 |
| плотность пара | 5.3 (vs air) |
| давление пара | 0.019-0.29Pa at 20-50℃ |
| показатель преломления | 1.5200 (estimate) |
| Fp | >230 °F |
| температура хранения | -20°C (or 4°C short term). |
| растворимость | H2O: 50 mg/mL, clear, colorless |
| пка | pK1:8.9 (25°C) |
| форма | Powder |
| цвет | White |
| РН | 4.0-6.0 (20-25℃, 0.1m in H2O) |
| Запах | Unpleasant Odor |
| Водородный показатель | 4 - 6 at 15,4 g/l at 25 °C |
| Биологические источники | synthetic |
| Растворимость в воде | freely soluble |
| λмакс | λ: 260 nm Amax: 0.400 λ: 280 nm Amax: 0.100 |
| Чувствительный | Air Sensitive |
| Мерк | 14,3376 |
| БРН | 1719757 |
| Стабильность | Stability Stable, but heat sensitive. Incompatible with strong oxidizing agents. Keep frozen at -20 to -10 C. |
| ИнЧИКей | VHJLVAABSRFDPM-UHFFFAOYSA-N |
| LogP | 0.07 at 25℃ and pH5 |
| Справочник по базе данных CAS | 3483-12-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | T8ID5YZU6Y |
| Справочник по химии NIST | 2,3-Butanediol, 1,4-dimercapto-, (r*,r*)-(3483-12-3) |
| Система регистрации веществ EPA | 1,4-Dithiothreitol (3483-12-3) |
| Поглощение | cut-off at 330nm |
| UNSPSC Code | 12352209 |
| NACRES | NB.22 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 25-36/37/38-22-20/21/22 | |||||||||
| Заявления о безопасности | 26-45-37/39-36 | |||||||||
| РИДАДР | UN 3335 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | EK1610000 | |||||||||
| F | 3-10-16-23 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29309099 | |||||||||
| Банк данных об опасных веществах | 3483-12-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
DL-Dithiothreitol химические свойства, назначение, производство
Химические свойства
Dithiothreitol (DTT), also known as Cleland's reagent, is a small-molecule redox reagent . Its oxidized form is a disulfide-bonded 6-membered ring. DTT has an epimeric ('sister') compound, dithioerythritol (DTE).DTT is a white crystalline powder. It is highly soluble in water (clear solution, OD<0.05 at 0.02M), but also in ethanol, chloroform, ether and ethyl acetate.
DTT is an unusually strong reducing agent, with a redox potential of -0.33 V at pH 7. The pKa of thiol groups is typically ~8.3.
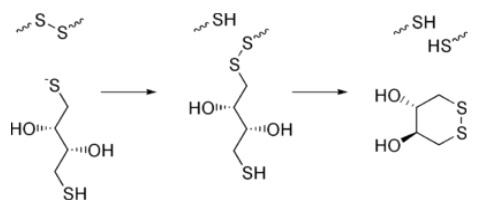
The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions.
Использование
DL-dithiothreitol (DTT) is a sulfhydryl compound that acts both as a reagent reducing disulfide bonds and as a protein denaturant on staphylococcal biofilm (Wu et al. 2011). Thanks to these properties, DTT is routinely used in clinical microbiology to liquefy respiratory specimens, but it has also been proven effective to detach biofilm from orthopaedic prosthesis (Drago et al. 2012, 2013). Since it is crucial to discriminate implant-related infections from aseptic loosening in orthopaedics, and because of the difficulty to diagnose subclinical infections, a prompt diagnosis of indwelling device-related infection is important for successful treatments (Borens et al. 2013). A fast microbiological diagnosis and bacterial identification should be recommended in order to set up a specific antimicrobial therapy. According to this strategy, the bacterial detachment from biofilm on explants should be accelerated by supporting the pathogen viability.Определение
ChEBI: 1,4-dithiothreitol is the threo-diastereomer of 1,4-dimercaptobutane-2,3-diol. It has a role as a reducing agent, a chelator and a human metabolite. It is a dithiol and a 1,4-dimercaptobutane-2,3-diol.прикладной
Dithiothreitol (DTT) is a water-soluble reducing reagent used for various applications in biotechnology, biology and biochemistry :reduces quantitatively disulfides, generating sulfhydryls (used typically at 1-10mM for protein SS reduction)
reduction of proteins before SDS-PAGE analysis, studies of protein structure and function (Kaji 1993)
keep sulfhydryl groups of biomolecules in the reduced state - protects biomolecules in various applications (enzymes or receptors, living cells under ionizing radiations)
prevents the fading of fluorescence such as FITC labeled conjugates (Picciolo 1984)
Общее описание
1,4-Dithiothreitol (DTT) is the threo isomer of 2,3-dihydroxy-1,4-dithiolbutane, and an isomer of 1,4-dithioerythritol. DTT is used in molecular biology to maintain sulfhydryl (-SH) groups in the reduced state and for quantitative reduction of disulfide (-S-S-) groups, as described by Cleland in his pioneering publication from the 1960's. Its usefulness as an reducing agent stems from its water solubility and reduced odor compared to previous thiol compounds.DTT is oxidized to the cyclic disulfide, and thereby ensures the reduction of other disulfides in solution. The disulfide reduction is complete in minutes at pH 8. DTT is less pungent and less toxic than 2-mercaptoethanol. Typically, a 7-fold lower concentration of DTT (100 mM) is required compared to 2-mercaptoethanol (5% v/v, 700 mM).
Синтез
The most widely used DL-Dithiothreitol (DTT) synthetic method is to be raw material with Soviet Union's moss sugar alcohol, use potassium permanganate oxidation earlier, obtains the sulfo-intermediate again under the thioacetic acid effect, and this sulfo-intermediate of hydrolysis obtains DTT at last.Yu discloses a synthesizing method of DL-Dithiothreitol.1,4-butylene glycol is used as an initiating raw material and undergoes an addition reaction with bromine at first to obtain 2,3-dibromo-1,4-butylene glycol; hydrolyzation under the catalyzation of alkali is carried out to obtain dioxirane; addition reaction with thioacetic acid is carried out to obtain dithiothreitol diacetate; and finally hydrolyzation under the catalyzation of alkali is carried out to obtain the DL-Dithiothreitol.
хранилище
Store lyophilized at 4°C, desiccated. In lyophilized form, the chemical is stable for 12 months. Once in solution, store at -20°C and use within 3 months to prevent loss of potency. Aliquot to avoid multiple freeze/thaw cycles.DTT solutions should be prepared fresh daily. If improperly stored (including room temperature and solution forms) its reducing ability may be reduced. Exposure to air should be minimized, even though DTT has a lower tendency to be oxidized directly by air than other reducing agents.
Recorded half-life of DTT solutions at various pH and temperatures (all are in M potassium phosphate buffer):
pH 6.5 @ 20°C= 40 hours
pH 7.5 @ 20°C = 10 hours
pH 8.5 @ 20°C = 1.4 hours
pH 8.5 @ 0°C = 11 hours
pH 8.5 @ 40°C = 0.2 hours
pH 8.5 @20°C (+0.1 mM Cu2+) = 0.6 hours
pH 8.5 @20°C (+0.1 mM EDTA) = 4 hours
DL-Dithiothreitol запасные части и сырье
DL-Dithiothreitol поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613681955282 | China | 311 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +1-2266000236 | Canada | 1641 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-029-81138252 +86-18789408387 |
China | 3889 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | ||
| +8619933239880 | China | 861 | 58 |