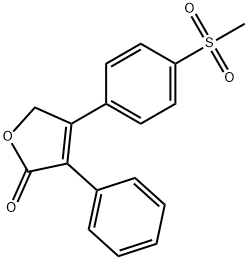
Рофекоксиб
- английское имяRofecoxib
- CAS №162011-90-7
- CBNumberCB6327130
- ФормулаC17H14O4S
- мольный вес314.36
- EINECS803-260-0
- номер MDLMFCD00935806
- файл Mol162011-90-7.mol
химическое свойство
| Температура плавления | 207°C |
| Температура кипения | 577.6±50.0 °C(Predicted) |
| плотность | 1.333±0.06 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | DMSO: soluble5mg/mL, clear (warmed) |
| форма | powder |
| цвет | white to beige |
| Растворимость в воде | 9mg/L(25 ºC) |
| Мерк | 14,8248 |
| Стабильность | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| Справочник по базе данных CAS | 162011-90-7(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | rofecoxib; Vioxx |
| FDA UNII | 0QTW8Z7MCR |
| Словарь наркотиков NCI | rofecoxib |
| Код УВД | M01AH02 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | LU5135000 | |||||||||
| кода HS | 2932.20.3000 | |||||||||
| Банк данных об опасных веществах | 162011-90-7(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Рофекоксиб химические свойства, назначение, производство
Описание
Rofecoxib ts a non-steroidal anti-inflammatory drug (NSAID) launched in Mexico, its first market, for the management of acute pain and the treatment of osteoarthritis (OA) and primary dysmenorrhea. Rofecoxib can be obtained by several different ways; one example is by arylation of a 4-bromofuranone with a phenylboronic acid under Suzuki conditions. Rofecoxib is a highly selective inhibitor of COX-2, the inducible isoform of cyclooxygenase and therefore exhibits a potent antiinflammatory activity without concomitant gastric or renal toxicities linked to the non-specific COX-1/2 inhibitors. In several clinical studies in patients with knee or hip osteoarthritis, Rofecoxib was evaluated at 12.5-50 mg doses once daily: it demonstrated efficacy for all primary and secondary endpoints at doses considerably weaker than those for classical non-specific NSAIDs, with good tolerance and less adverse effects. Selective COX-2 inhibitors potentially have a large spectrum of activity including new indications such as Alzheimer's disease, colorectal cancer, irritable bowel disease or urinary incontinence.Химические свойства
Off-White (Pale Yellow) Crystalline PowderИспользование
Rofecoxib has been used in high performance bioaffinity chromatography.Определение
ChEBI: A butenolide that is furan-2(5H)-one that is substituted by a phenyl group at position 3 and by a p-(methylsulfonyl)phenyl group at position 4. A selective cyclooxygenase 2 inhibitor, it was used from 1999 to 2004 for the tr atment of ostoarthritis, but was withdrawn following concerns about an associated increased risk of heart attack and stroke.Показания
Rofecoxib is approved for the treatment of osteoarthritis, dysmenorrhea, and acute pain. The most common adverse reactions to rofecoxib are mild to moderate GI irritation (diarrhea, nausea, vomiting, dyspepsia, abdominal pain). Lower extremity edema and hypertension occur relatively frequently (about 3.5%). It is not metabolized by CYP2C9, so rofecoxib should not be subject to some of the interactions seen with celecoxib. However, its metabolism is increased by the coadministration of rifampin, which acts as a nonspecific inducer of hepatic metabolism.Механизм действия
Rofecoxib is excreted primarily in the urine (72%) as metabolites. Less than 1% is excreted in the urine as unchanged drug, whereas approximately 14% is excreted in the feces as unchanged drug. Although the metabolism of rofecoxib has not been fully determined, the microsomal cytochrome P450 system appears to play only a minor role—a major difference in the metabolic routes of rofecoxib and celecoxib. The major metabolic route appears to form reduction of the dihydrofuranone ring system by cystolic enzymes to the to cis- and trans- dihydro derivatives. Also isolated is the glucuronide of a hydroxy derivative that results from CYP2C9 oxidative metabolism. None of the isolated metabolites of rofecoxib possess pharmacological activity as COX-1 or COX-2 inhibitors.Фармакокине?тика
Rofecoxib has been synthesized by a number of synthetic routes that have been summarized elsewhere. It was the second selective COX-2 inhibitor to be marketed. Rofecoxib is well absorbed from the GI tract on oral administration, with peak plasma levels generally being attained within 2 to 3 hours of dosing. Bioavailability averages 93% following administration of a single dose. The area under the plasma concentration–time curve is increased in patients older than 65 years compared to younger adults and is increased slightly in black and Hispanic patients compared with white patients, but the difference is not considered to be clinically significant.Клиническое использование
Rofecoxib was indicated for the relief of the signs and symptoms of osteoarthritis, for the management of acute pain in adults, and for the treatment of primary dysmenorrhea.Синтез
Rofecoxib can be obtained by different synthetic routes, e.g., by condensation of phenylacetic acid with ethyl bromoacetate to ethyl 2-phenylacetoxyacetate, which is then cyclized to a hydroxyfuranone. Subsequently, the hydroxyfuranone reacts with trifluoromethanesulfonic (triflic) anhydride to the corresponding triflate which reacts with LiBr to yield a bromofuranone. The bromofuranone is condensed with 4- (methylsulfanyl)phenylboronic acid to give 4-[4-(methylsulfanyl)phenyl]-3-phenylfuran- 2(5H)-one which is finally oxidized to rofecoxib.Рофекоксиб запасные части и сырье
Рофекоксиб поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-17332992504 +86-17332992504 |
China | 300 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| 13480692018 | CHINA | 954 | 58 |