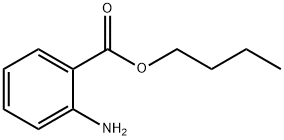
BUTYL ANTHRANILATE
- русский язык имя
- английское имяBUTYL ANTHRANILATE
- CAS №7756-96-9
- CBNumberCB5317594
- ФормулаC11H15NO2
- мольный вес193.24
- EINECS231-816-7
- номер MDLMFCD00036506
- файл Mol7756-96-9.mol
химическое свойство
| Температура плавления | <0 °C |
| Температура кипения | 303 °C(lit.) |
| плотность | 1.06 g/mL at 25 °C(lit.) |
| показатель преломления | n |
| FEMA | 2181 | BUTYL ANTHRANILATE |
| Fp | >230 °F |
| пка | 2.17±0.10(Predicted) |
| цвет | A colourless or very pale straw-coloured liquid. |
| Запах | at 100.00 %. petitgrain plum sweet grape fruity wine floral berry powdery |
| Odor Type | fruity |
| Номер JECFA | 1536 |
| Стабильность | Air and light sensitive. Incompatible with alkalies, acids, strong oxidizing agents. |
| LogP | 3.69 at 23℃ and pH8.5 |
| Справочник по базе данных CAS | 7756-96-9(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | BUTYL ANTHRANILATE |
| FDA 21 CFR | 172.515 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 73DAT3M81O |
| Система регистрации веществ EPA | Butyl anthranilate (7756-96-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36/37/38 | |||||||||
| Заявления о безопасности | 26 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | DG1527000 | |||||||||
| Класс опасности | IRRITANT | |||||||||
| кода HS | 2922493800 | |||||||||
| Токсичность | Both the acute oral LD50 value in rats and the acute dermal LD50 value in rabbits exceeded 5 g/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
BUTYL ANTHRANILATE химические свойства, назначение, производство
Химические свойства
Butyl anthranilate has a sweet, faint, fruit (plum, petigrain) note.Вхождение
Reported present in peppermint oil from Brazil, Achillea ageratum, tea and in apple aroma.Подготовка
Prepared by transesterification of methyl anthranilate (methyl 2-aminobenzoate) with n-butyl alcohol in the presence of HCl.Определение
ChEBI: A benzoate ester obtained by formal condensation of the carboxy group of anthranilic acid with the hydroxy group of butanol. Found in several fruit species, it is used as a flavouring and fragrance agent and also exhibits insect repellent properties.Общее описание
Dark brown liquid. Insoluble in water.Реакции воздуха и воды
Sensitive to air and light. Insoluble in water. BUTYL ANTHRANILATE will hydrolyze under high and low pH conditions. .Профиль реактивности
BUTYL ANTHRANILATE is an aminophenyl ester derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.Пожароопасность
Flash point data for BUTYL ANTHRANILATE are not available. BUTYL ANTHRANILATE is probably combustible.Метаболизм
Esters of benzoic acid are presumably either hydrolysed and then metabolized according to the normal pattern for the alcohol and acid produced, or possibly in some cases the ring may be hydroxylated and the product excreted as a glucuronide or sulphate ester . H-Butanol and isobutanol are rapidly oxidized in vivo, presumably to the aldehyde and acid ; only small amounts were excreted by rabbits as the glucuronic acid conjugatesBUTYL ANTHRANILATE запасные части и сырье
сырьё
BUTYL ANTHRANILATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8617013299288 | China | 12373 | 58 | |
| 15957180504 | China | 193 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 |