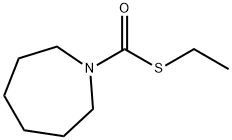
Molinate
- русский язык имя
- английское имяMolinate
- CAS №2212-67-1
- CBNumberCB4768126
- ФормулаC9H17NOS
- мольный вес187.3
- EINECS218-661-0
- номер MDLMFCD00055352
- файл Mol2212-67-1.mol
| Температура плавления | <25 °C |
| Температура кипения | 202°C (10 mmHg) |
| плотность | 1.06 |
| показатель преломления | 1.5250 (estimate) |
| Fp | 100 °C |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| пка | -1.22±0.20(Predicted) |
| форма | Liquid |
| цвет | Amber |
| Растворимость в воде | 0.08 g/100 mL |
| БРН | 1239196 |
| LogP | 3.210 |
| Справочник по базе данных CAS | 2212-67-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-5 |
| FDA UNII | 68N5G08DJQ |
| Предложение 65 Список | Molinate |
| Справочник по химии NIST | Molinate(2212-67-1) |
| Пестициды Закон о свободе информации (FOIA) | Molinate |
| Система регистрации веществ EPA | Molinate (2212-67-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,N,Xn |
| Заявления о рисках | 20/22-40-43-48/22-63-50/53-62 |
| Заявления о безопасности | 36/37-46-60-61 |
| РИДАДР | 2902 |
| WGK Германия | 3 |
| RTECS | CM2625000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 2212-67-1(Hazardous Substances Data) |
| Токсичность | LC50 (96-hour) for bluegill sunfish 29–30 mg/L, goldfish 30 mg/L, mosquito fish 16.4 mg/L, rainbow trout 0.2–1.3 mg/L (Hartley and Kidd, 1987); acute oral LD50 of technical molinate for rats and mice 720 and 795 mg/kg, respectively (Ashton and Monaco, 1991), 501 mg/kg (RTECS, 1985). |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H302+H332:Вредно при проглатывании или при вдыхании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Molinate химические свойства, назначение, производство
Использование
Selective herbicide used to control the germination of annual grasses and broadleaved weeds in rice crops.Общее описание
Clear liquid with aromatic odor. Non corrosive. Used as an herbicide.Реакции воздуха и воды
Water soluble. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.Профиль реактивности
Molinate is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.Сельскохозяйственное использование
Herbicide: Molinate is a selective herbicide used on rice for the control of water grass and other weeds.Торговое название
ARROSOLO®; FELAN®; HIGALNATE®; HYDRAM®; JALAN®; MALERBANE-GIAVONI-L®;ORDAM®; ORDRAM®; R-4572®; RICECO; SAKKIMOL®; STAUFFER R 4,572®; YALAN®; YULAN®Экологическая судьба
Soil. Hydrolyzes in soil forming ethyl mercaptan, carbon dioxide and dialkylamine (half-life approximately 2–5 weeks) (Hartley and Kidd, 1987). At recommended rates of application, the half-life of molinate in moist loam soils at 21–27°C is approximately 3 weeks (Humburg et al., 1989). Rajagopal et al. (1989) reported that under flooded conditions, molinate was hydroxylated at the 3- and 4-position with subsequent oxidation forming many compounds including molinate sulfoxide, carboxymethyl molinate, hexahydroazepine- 1-carbothioate, 4-hydroxymolinate, 4-hydroxymolinate sulfoxide, hexahydroazepine, S-methyl hexahydroazepine-1-carbothioate, 4-ketomolinate, 4-hydroxyhexahydroazepine, 4-hydroxy-N-acetyl-hexahydroazepine, carbon dioxide and bound residues.Plant. Molinate is rapidly metabolized by plants releasing carbon dioxide and naturally occurring plant constituents (Humburg et al., 1989).
Photolytic. Molinate in a hydrogen peroxide solution (120 mM) was irradiated by UV light (l = 290 nm) at 23°C. The major photooxidation products were the two isomers of 2-oxomolinate (20% yield) and s-molinate oxide (5% yield) (Draper and Crosby, 1984).
Half-lives of 180 and 120 hours were observed using one and two equivalents of hydrogen peroxide, respectively (Draper and Crosby, 1984). Molinate has a UV absorption maximum at 225 nm and no absorption at wavelengths >290 nm. Therefore, molinate is not expected to undergo aqueous photolysis under natural sunlight (l = 290 nm). In the presence of tryptophan, a naturally occurring photosensitizer, molinate in aqueous solution photodegraded to form 1-((ethylsulfinyl)carbonyl)hexahydro-1H-azepine, S-ethyl hexahydro-2- oxo-1H-azepine-1-carbothioate and hexamethyleneimine (Soderquist et al., 1977).
Chemical/Physical. Metabolites identified in tap water were molinate sulfoxide, 3- and 4-hydroxymolinate, ketohexamethyleneimine and 4-ketomolinate (Verschueren, 1983).
Метаболический путь
Juvenile white sturgeon and common carp are exposed to 14C-molinate in a flow-through metabolism system and oxidize molinate to form several products and hydrolyze or conjugate with glutathione (GSH), the sulfoxide, or sulfone. Both fish form a D-glucuronic acid conjugate. The higher toxicity of molinate in common carp may be due to greater bioconcentration, slower depuration, and less efficient metabolic deactivation. In the blood of common carp, molinate is oxidized by erythrocytes to the sulfoxide and possibly the sulfone, then conjugated with GSH or cysteine and cleaved to form mercapturic acid in both erythrocytes and plasma. Conjugation and possible hemoglobin carbamylation occur only after sulfoxidation of molinate. Molinate is distributed uniformly throughout the soil layers, and its degradation products are identified as 2-oxomolinate, 4-oxomolinate, molinate acid, and hexamethyleneimine. In rice plants, 4-hydroxymolinate, 2-oxomolinate, 4-oxomolinate, S-ethyl-N-carboxymethylthiocarbamate, molinate acid, and molinate alcohol are detected. By the soil microorganisms, oxidation of the S-ethyl moiety is considered to be the main pathway, and hydroxy and oxoderivatives on the azepine ring are identified.Molinate запасные части и сырье
Molinate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-057181025280; +8617767106207 |
China | 49734 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +8618950047208 | China | 43416 | 58 |