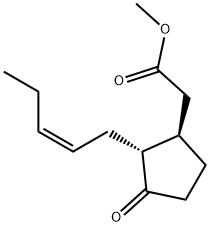
METHYL JASMONATE
- русский язык имя
- английское имяMETHYL JASMONATE
- CAS №1211-29-6
- CBNumberCB4100148
- ФормулаC13H20O3
- мольный вес224.3
- EINECS214-918-6
- номер MDLMFCD00151382
- файл Mol1211-29-6.mol
химическое свойство
| альфа | D -76.5° (c = 3.4 in CH3OH) |
| Температура кипения | 110 °C0.2 mm Hg(lit.) |
| плотность | 1.03 g/mL at 25 °C(lit.) |
| FEMA | 3410 | METHYL JASMONATE |
| показатель преломления | n |
| Fp | >230 °F |
| температура хранения | Sealed in dry,Room Temperature |
| растворимость | Almost insoluble in water, soluble in alcohol and oils. |
| форма | oily liquid |
| Удельный вес | 1.02 |
| цвет | Colorless |
| Запах | at 100.00 %. floral fresh petal magnolia oily waxy |
| Odor Type | floral |
| Номер JECFA | 1400 |
| LogP | 2.12 |
| Справочник по базе данных CAS | 1211-29-6(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | METHYL JASMONATE |
| FDA UNII | 900N171A0F |
| Система регистрации веществ EPA | Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)- (1211-29-6) |
больше
| WGK Германия | 3 |
| Банк данных об опасных веществах | 1211-29-6(Hazardous Substances Data) |
METHYL JASMONATE химические свойства, назначение, производство
Описание
Methyl jasmonate has a powerful, floral-herbaceous, sweet, persistent odor. Can be isolated from jasmine oil; or it may be prepared synthetically (probably in the trans-form) from muconic acid via the methyl-3-oxo-cyclopenthyl acetate.Химические свойства
METHYL JASMONATE is a volatile component of jasmine flower absolute. It is a colorless to pale yellow liquid, bp0.125 kPa 116–118 °C, d20 20 1.022–1.028, n20 D 1.473–1.477, possessing an odor reminiscent of the floral heart of jasmine. Synthesis of the title compound is accomplished by reaction of (2Z)-2-buten-1-yl bromide with Li in the presence of copper-(I) iodide, and the product is treated with a mixture of 2-methylene- and 4-methylene-3-oxocyclopentylacetic acid methyl ester. The aforementioned ester mixture is obtained by reaction of methyl 3-oxocyclopentylacetate with formaldehyde.An alternative route uses a palladium-catalyzed decarboxylation of the readily available substituted allyl 2-oxocyclopentanecarboxylates, which leads to the corresponding cyclopentenones. Addition of dimethylmalonate, with subsequent saponification/decarboxylation and, if necessary, (Z)-selective hydrogenation, yields methyl jasmonate:
Methyl jasmonate exists in an equilibrium mixture in which the trans-isomer dominates. But of all possible four enantiomeric isomers, chiefly the minor (+)-cis-isomer is responsible for the typical sensory properties of methyl jasmonate. Therefore, several approaches to obtain this material selectively have been developed either by directed syntheses or by enrichment using special isomerization distillation techniques.
Methyl jasmonate is used in fine fragrances where it provides rich, soft effects in jasmine and muguet compositions.
Вхождение
Reportedly identified in jasmine oil (Jasminum grandiflorum L.) and in Tunisian rosemary. Also found in lemon peel oil, peppermint oil and green and fermented teaИспользование
Methyl ester in perfumes.Определение
ChEBI: A jasmonate ester that is the methyl ester of jasmonic acid.Торговое название
Jasmoneige® (Nippon Zeon), Splendione® (Firmenich)METHYL JASMONATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5886 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615318402391 | China | 809 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |