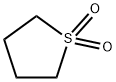
СУЛЬФОЛАН
- английское имяSulfolane
- CAS №126-33-0
- CBNumberCB3852996
- ФормулаC4H8O2S
- мольный вес120.17
- EINECS204-783-1
- номер MDLMFCD00005484
- файл Mol126-33-0.mol
| Температура плавления | 20-26 °C (lit.) |
| Температура кипения | 104 °C/0.2 mmHg (lit.) 285 °C (lit.) |
| плотность | 1.261 g/mL at 25 °C (lit.) |
| плотность пара | 4.2 (vs air) |
| давление пара | 0.01 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 330 °F |
| температура хранения | Store below +30°C. |
| растворимость | >=100g/l soluble |
| форма | Liquid After Melting |
| цвет | Clear yellow |
| Растворимость в воде | soluble |
| Точка замерзания | 26℃ |
| Чувствительный | Hygroscopic |
| Мерк | 14,8955 |
| БРН | 107765 |
| Диэлектрическая постоянная | 44.0 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | HXJUTPCZVOIRIF-UHFFFAOYSA-N |
| LogP | 0 at 20℃ |
| Справочник по базе данных CAS | 126-33-0(CAS DataBase Reference) |
| FDA UNII | Y5L06AH4G5 |
| Справочник по химии NIST | Thiophene, tetrahydro-, 1,1-dioxide(126-33-0) |
| Система регистрации веществ EPA | Sulfolane (126-33-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| Заявления о безопасности | 23-25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | XN0700000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 126-33-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 1.54 ml/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
СУЛЬФОЛАН химические свойства, назначение, производство
Химические свойства
Sulfolane is a colorless oily liquidИспользование
Sulfolane is used in lithium-ion batteries.Общее описание
Colorless oily liquid with a weak oily odor. Solidifies (freezing point is 79°) and sinks on first contact with water, then mixes with water. F.Реакции воздуха и воды
Water soluble.Профиль реактивности
Mixing Sulfolane in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid and oleum [NFPA 1991]. With nitrating agents (nitronium tetrafluoroborate in Sulfolane) very highly exothermic reactions are known to occur, [J. Org. Chem., 1978, 43, 4677].Опасность
Combustible. Toxic by ingestion.Угроза здоровью
Very mildly irritating to the eyes.Пожароопасность
Special Hazards of Combustion Products: Toxic, irritating gases may be generated in fires.Промышленное использование
Sulfolane is the most common commercially available sulfone solvent. The solvent, also known as tetrahydrothiophene-1,1-dioxide, is a colorless, highly polar liquid consisting of a fully hydrogenated five-member sulfur-carbon heterocyclic thiophene ring. The solvent is available as both anhydrous sulfolane and as sulfolane containing 3 wt% deionized water. Sulfolane is used as a reaction medium, as a solvent for a wide variety of organic chemicals and polymers, and as an extraction solvent.Sulfolane is a very high boiling point, colorless liquid with a very high viscosity (10.3 centipoises) and medium surface tension value (35.5 dynes/cm).Sulfolane is miscible with water and many organic solvents.
Sulfolane is used to separate aromatic hydrocarbons from aliphatic hydrocarbons. The extraction process first developed by Shell Oil in 1959 and which is referred to as the "Sulfolane" process is used worldwide. The solvency of sulfolane for certain fatty acids and fatty acid esters is the basis for upgrading animal and vegetable fatty acids used in food products, paints, plastics, resins, and soaps.
Sulfolane is used to remove acidic components like hydrogen sulfide and carbon dioxide from gas feed stocks. Sulfolane is used as a polymerization solvent for the production of polysulfones, polysiloxanes, polyphenylene ethers, and other polymers. Sulfolane is said to increase the reaction rates, afford easier polymer purification, and improved thermal stability. Sulfolane is a solvent for dissolving a variety of polymers for use in the fiberspinning process.Cellulose and cellulose ester polymers can be plasticized with sulfolane to give improved flexibility and other physical property improvements. Other application areas that have used sulfolane include electronic and electrical uses, textile dye uses, curing of polysulfide sealant, and as a catalyst in certain synthetic reactions.
Возможный контакт
Sulfolane is used primarily as a process solvent for extraction of aromatics and for purification of acid gases. Used as a curing agent for epoxy resins, in medicine ash an antibacterial; fractionation of wood tars, tall oil, and other fatty acids; a component of hydraulic fluid; in textile finishing.Канцерогенность
Sulfolane was not mutagenic in bacterial assays with or without metabolic activation.Перевозки
UN3334 Aviation regulated liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material. Technical Name Required.Методы очистки
prepared commercially by a Diels-Alder reaction of between 1,3-butadiene and sulfur dioxide, followed by Raney nickel hydrogenation. The principal impurities are water, 3-sulfolene, 2-sulfolene and 2-isopropyl sulfolanyl ether. It is dried by passage through a column of molecular sieves. Distil it under reduced pressure through a column packed with stainless steel helices. Again dry it with molecular sieves and distil. [Cram et al. J Am Chem Soc 83 3678 1961, Coetzee Pure Appl Chem 49 211 1977.] Alternatively, it is stirred at 50o, and small portions of solid KMnO4 are added until the colour persists during 1hour. Dropwise addition of MeOH then destroys the excess KMnO4; the solution is filtered, freed from potassium ions by passage through an ion-exchange column and dried under vacuum. It has also been distilled in a vacuum from KOH pellets. It is hygroscopic. [See Sacco et al. J Phys Chem 80 749 1976, J Chem Soc, Faraday Trans 1 73 1936 1977, 74 2070 1978, Trans Faraday Soc 62 2738 1966.] Coetzee has reviewed the methods of purification of sulfolane, and also the removal of impurities. [Coetzee in Recommended Methods of Purification of Solvents and Tests for Impurities, Coetzee Ed. Pergamon Press, 1982, Beilstein 17 I 5, 17 III/IV 37, 17/1 V 39.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with nitronium tetrafluoroborate(1-) is potentially explosive.СУЛЬФОЛАН запасные части и сырье
сырьё
1of2
запасной предмет
- 1-Нафталинсульфонилхлорид
- N,N-диметилметилениминий иодид
- Desulfurizer,high efficiency
- ГЕКСАФЛУМУРОН
- Cefteram pivoxil
- 4-AMINO-2,6-DIFLUOROPYRIMIDINE
- (4-фторфенил)метансульфанил хлорид
- (3-фторфенил)метансульфанил хлорид
- Chlorfluazuron
- 9,10-ANTHRACENEDICARBOXALDEHYDE
1of4
СУЛЬФОЛАН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13806087780 | China | 17365 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8615713292910 | China | 341 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8615373025980 | China | 895 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 |
СУЛЬФОЛАН Обзор)
1of4