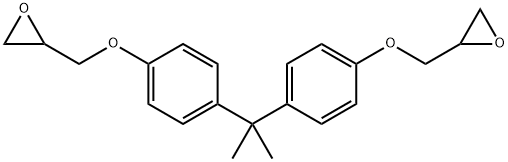
Бисфенола А диглицидиловый эфир
- английское имяBISPHENOL A DIGLYCIDYL ETHER RESIN
- CAS №1675-54-3
- CBNumberCB3749115
- ФормулаC21H24O4
- мольный вес340.41
- EINECS216-823-5
- номер MDLMFCD00080480
- файл Mol1675-54-3.mol
| Температура плавления | 40-44 °C |
| Температура кипения | 210 °C / 1mmHg |
| плотность | 1,17 g/cm3 |
| давление пара | 0Pa at 25℃ |
| показатель преломления | 1.5735 |
| температура хранения | Inert atmosphere,2-8°C |
| растворимость | Soluble in DMSO (up to 30 mg/ml) or in Ethanol (up to 15 mg/ml) |
| форма | viscous liquid |
| цвет | White |
| Растворимость в воде | Soluble in 100% ethanol, dimethyl sulfoxide (100 mM), dimethyl formamide, chloroform, methanol, and ethanol (50 mM). Insoluble in water. |
| БРН | 299026 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| LogP | 3.242 at 25℃ |
| Справочник по базе данных CAS | 1675-54-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6 |
| FDA UNII | F3XRM1NX4H |
| МАИР | 3 (Vol. 47, 71) 1999 |
| Система регистрации веществ EPA | Bisphenol A diglycidyl ether (1675-54-3) |
| UNSPSC Code | 85151701 |
| NACRES | NA.77 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/38-43 | |||||||||
| Заявления о безопасности | 28-37/39 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TX3800000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2910900090 | |||||||||
| Банк данных об опасных веществах | 1675-54-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in rabbit: 1980mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Бисфенола А диглицидиловый эфир химические свойства, назначение, производство
Описание
Most epoxy resins result from polymerization of bisphenol A diglycidyl ether (BADGE). Delayed hypersensitivity is caused by the low-molecular-weight monomer BADGE (MW 340 g/mol), the dimer having a much lower sensitization power. This allergen caused contact dermatitis in six workers in a plant producing printed circuits boards made of copper sheets and fiberglass fabric impregnated with a brominated epoxy resin. It can also be contained in adhesives.Химические свойства
Diglycidyl ether of bisphenol A is a colorless to light amber liquid. Slight epoxy odor.Использование
Bisphenol A derivative. A PPARγ antagonist; exhibits estrogenic activity; An inhibitor of PPARγ and suppressor of TNFα.Методы производства
The synthesis of the basic epoxy resin molecule involves the reaction of epichlorohydrin with bisphenol A, the latter requiring two basic intermediates for synthesis, acetone and phenol. Theoretically, the production of the bisphenol A diglycidyl ether requires 2 mol of epichlorohydrin for each mole of the phenol. Epoxy resins of higher molecular weight are obtained by reducing the epichlorohydrin/bisphenol A ratio. This reaction involves consumption of the initial epoxy groups in the epichlorohydrin and of some of the groups formed by dehydrohalogenation.Общее описание
Odorless yellowish brown liquid. Sinks in water.Реакции воздуха и воды
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164]. Insoluble in water.Профиль реактивности
Epoxides, such as BISPHENOL A DIGLYCIDYL ETHER RESIN, are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.Угроза здоровью
Contact with liquid irritates eyes. Prolonged or repeated contact with skin causes irritation and dermatitis.Пожароопасность
BISPHENOL A DIGLYCIDYL ETHER RESIN is probably combustible.Биологическая активность
PPAR γ pure antagonist with micromolar affinity in 3T3-L1 and 3T3-F442A preadipocyte cells; selective over PPAR δ and PPAR α . Antagonizes the ability of rosiglitazone to stimulate transcriptional activity of PPAR γ . Acts as a PPAR γ agonist in an ECV304 cell line. Also produces PPARg-independent apoptosis of tumor cells via several mechanisms. Active in vivo .Контактные аллергены
Most epoxy resins result from polymerization of bisphenol A diglycidyl ether (BADGE). Delayed hypersensitivity is caused by the low-molecular-weight monomer BADGE (Molecular Weight 340 g/mol), the dimer having much a lower sensitization power. This allergen caused contact dermatitis in six workers in a plant producing printed circuits boards made of copper sheets and fiber glass fabric impregnated with a brominated epoxy resin. It can be contained in adhesives.Возможный контакт
Diglycidyl ether of bisphenol A is used as a basic active ingredient of epoxy resins.Канцерогенность
In summary, a number of carcinogenicity studies involving the topical application of pure BADGE as well as EPON Resin 828 and other commercial BADGE-based resins have been carried out in experimental animals.Viewing the studies as a whole, the weight of evidence does not show that BADGE or BADGE-based epoxy resins are carcinogenic.Несовместимости
Easily oxidized in air; presumed to form unstable and explosive peroxides in storage. Incompatible with strong acids; strong oxidizers.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.Бисфенола А диглицидиловый эфир запасные части и сырье
Бисфенола А диглицидиловый эфир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29808 | 58 | |
| +8618530059196 | China | 12114 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32159 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 15369953316 +8615369953316 |
China | 2123 | 58 |