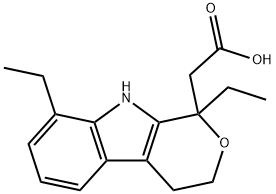
Этодолак
- английское имяEtodolac
- CAS №41340-25-4
- CBNumberCB3216064
- ФормулаC17H21NO3
- мольный вес287.35
- EINECS629-689-1
- номер MDLMFCD00133313
- файл Mol41340-25-4.mol
химическое свойство
| Температура плавления | 145-1480C |
| Температура кипения | 507.9±45.0 °C(Predicted) |
| плотность | 1.193±0.06 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water, freely soluble in acetone and in ethanol (96 per cent) |
| пка | 4.65(at 25℃) |
| форма | Solid |
| цвет | White to Orange to Green |
| Растворимость в воде | 40mg/L(37 ºC) |
| Мерк | 14,3874 |
| BCS Class | 2 |
| ИнЧИКей | NNYBQONXHNTVIJ-QGZVFWFLSA-N |
| Справочник по базе данных CAS | 41340-25-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 2M36281008 |
| Предложение 65 Список | Etodolac |
| Словарь наркотиков NCI | etodolac |
| Код УВД | M01AB08 |
| Система регистрации веществ EPA | Pyrano[3,4-b]indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro- (41340-25-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
больше
| Коды опасности | T,Xi | |||||||||
| Заявления о рисках | 23/24/25-40-36-25-36/37/38 | |||||||||
| Заявления о безопасности | 22-36-45-26-36/37 | |||||||||
| РИДАДР | 3249 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UQ0360000 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29349990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Этодолак химические свойства, назначение, производство
Описание
Etodolac (etodolic acid) is a non-steroidal antiinflammatory/analgesic agent useful in the treatment of various inflammatory conditions, including rheumatoid and osteoarthritis.Химические свойства
Crystalline SolidИспользование
Etodolac is a non-steroidal anti-inflammatory drug (NSAID) that selectively inhibits cyclooxygenase-2 (COX-2). Etodolac displays anti-inflammatory effects in both adjuvant arthritic and normal rats. E todolac is an anti-inflammatory; analgesic.Определение
ChEBI: A monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl moiety. A preferential inhibitor of cyclo-oxygenase 2 and non-steroidal anti-inflammatory, it is used for the treatment of rheumatoid arthritis and osteoarthritis, and for the alleviation of postoperative pain. Administered as the racemate, only the (S)-enantiomer is active.Показания
Etodolac (Lodine) is indicated for the treatment of osteoarthritis, rheumatoid arthritis, and acute pain. It inhibits COX-2 with slightly more selectivity than COX-1 and therefore produces less GI toxicity than many other NSAIDs. Common adverse effects include skin rashes and CNS effects.Общее описание
Etodolac (Lodine, Ultradol), a chiral, COX-2 selectiveNSAID drug that is marketed as a racemate, possesses an indolering as the aryl portion of this group of NSAID drugs.It shares many similar properties of this group and is indicatedfor short- and long-term management of pain and OA.Similar to ketorolac, etodolac exhibits several uniqueenantioselective pharmacokinetic properties. For example,the “inactive” (R)-enantiomer has approximately a 10-foldhigher plasma concentration than the active (S)-enantiomer.Furthermore, the active (S)-enantiomer is less protein boundthan its (R)-enantiomer and therefore has a very large volumeof distribution. It is well absorbed with an elimination halflifeof 6 to 8 hours. Etodolac is extensively metabolized intothree major inactive metabolites, 6-hydroxy etodolac (via aromatichydroxylation), 7-hydroxy-etodolac (via aromatic hydroxylation),and 8-(1'-hydroxylethyl) etodolac (via benzylichydroxylation), which are eliminated as the correspondingether glucuronides. Its unstable, acyl glucuronide, however,is subject to enterohepatic circulation and reactivation tothe parent drug, similar to other NSAIDS in this class.
Биологическая активность
Non-steroidal anti-inflammatory drug (NSAID) that selectively inhibits cyclooxygenase-2 (COX-2) (IC 50 values are 53 and >100 μ M for COX-2 and COX-1 respectively). Displays anti-inflammatory effects in both adjuvant arthritic and normal rats.Механизм действия
The primary mechanism of action appears to be inhibition of the biosynthesis of prostaglandins at the cyclooxygenase step, with no inhibition of the lipoxygenase system. Etodolac, however, possesses a more favorable ratio of inhibition of prostaglandin biosynthesis in human rheumatoid synoviocytes and chondrocytes than by cultured human gastric mucosal cells compared to ibuprofen, indomethacin, naproxen, diclofenac, and piroxicam.Фармакокине?тика
Etodolac is rapidly absorbed following oral administration, with maximum serum levels being achieved within 1 to 2 hours, and it is highly bound to plasma proteins (99%) with pKa 4.7. The penetration of etodolac into synovial fluid is greater than or equal to that of tolmetin, piroxicam, or ibuprofen. Only diclofenac appears to provide greater penetration.Клиническое использование
Etodolac is promoted as the first of a new chemical class of anti-inflammatory drugs, the pyranocarboxylic acids. Although not strictly an arylacetic acid derivative (because there is a two-carbon atom separation between the carboxylic acid function and the hetero-aromatic ring), it still possesses structural characteristics similar to those of the heteroarylacetic acids and is classified here. It was introduced in the United States in 1991 for acute and long-term use in the management of osteoarthritis and as an analgetic. It also possesses antipyretic activity. Etodolac is marketed as a racemic mixture, although only the S-(+)-enantiomer possesses anti-inflammatory activity in animal models. Etodolac also displays a high degree of enantioselectivity in its inhibitory effects on the arachidonic acid cyclooxygenase system.Метаболизм
Etodolac is metabolized to three hydroxylated metabolites and to glucuronide conjugates, none of which possesses important pharmacological activity. Metabolism appears to be the same in the elderly as in the general population, so no dosage adjustment appears necessary. Etodolac is indicated for the management of the signs and symptoms of osteoarthritis and for the management of pain.Этодолак запасные части и сырье
Этодолак поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-13131129325 | China | 5887 | 58 | |
| +8613043004613 | China | 283 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-21-33585366 - 03@ | CHINA | 738 | 60 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 86+18663751872 | CHINA | 491 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 |