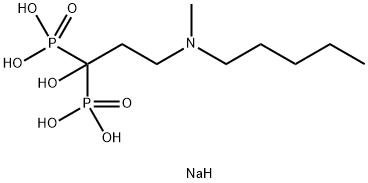
Ибандронат натрия
- английское имяIbandronate sodium
- CAS №138844-81-2
- CBNumberCB31261504
- ФормулаC9H24NNaO7P2
- мольный вес343.23
- EINECS682-157-0
- номер MDLMFCD07197214
- файл Mol138844-81-2.mol
химическое свойство
| температура хранения | 2-8°C |
| растворимость | H2O: >10mg/mL |
| форма | solid |
| цвет | white |
| Растворимость в воде | H2O: >10mg/mL |
| Мерк | 14,4873 |
| Стабильность | Hygroscopic |
| ИнЧИКей | LXLBEOAZMZAZND-UHFFFAOYSA-M |
| FDA UNII | 23Y0B94E49 |
| UNSPSC Code | 41106300 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Ибандронат натрия химические свойства, назначение, производство
Описание
ibandronate sodium (BONIVA) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl) amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, monohydrate with the molecular formula C9H22NO7P2Na?H20 and a molecular weight of 359.24. Ibandronate sodium is a white-to off-white powder. It is freely soluble in water and practically insoluble in organic solvents.BONIVA is available as a white, oblong, 2.5-mg film-coated tablet for daily oral administration or as a white, oblong, 150-mg film-coated tablet for once-monthly oral administration. One 2.5-mg film-coated tablet contains 2.813 mg ibandronate monosodium monohydrate, equivalent to 2.5 mg free acid. One 150-mg film-coated tablet contains 168.75 mg ibandronate monosodium monohydrate, equivalent to 150 mg free acid. BONIVA also contains the following inactive ingredients: lactose monohydrate, povidone, microcrystalline cellulose, crospovidone, purified stearic acid, colloidal silicon dioxide, and purified water. The tablet film coating contains hypromellose, titanium dioxide, talc, polyethylene glycol 6000, and purified water.
Использование
Ibandronate sodium salt has been used to study its effect on the proliferation and ultrastructure of Leishmania and Giardia by the generation of concentration curves. It has also been used to elucidate the route by which nitrogen-containing bisphosphonates (N-BPs) enter the cytosol and inhibit their molecular target.Фармакокине?тика
Its mechanism of action is identical to the other bisphosphonate agents. Administered daily (2.5 mg), ibandronate has been clinically shown to reduce the risk of vertebral fractures by 62%. If administered on an intermittent basis (20 mg), it reduces the risk of vertebral fractures by 50%. Ibandronate (2.5 mg daily), along with 500 mg of supplemental calcium, has been clinically shown to increase BMD in the hip (1.8%), femoral neck (2.0%), and lumbar spine (3.1%). The 150-mg formulation approved in March 2005 represents the first oral therapy for a chronic disease to be administered once monthly.Клиническое использование
Ibandronate sodium was approved in May 2003 for the treatment and prevention of osteoporosis in postmenopausal women.Побочные эффекты
Adverse events as sociated with the injectable form ulation included arthralgia, back and abdominal pain, and hypertens ion. There is a risk of renal toxicity that is inversely related to the rate of administration of this formulation.Метаболизм
The oral bioavailability of this agent is extremely poor (0.6%) and is adversely affected by the presence of food, beverages other than water, and other medications, including calcium or vitamin D supplements and antacids. Because of the increased calcium content in mineral water, patients should not take this medication with this type of water. Drugs that inhibit gastric acid secretion (e.g., H2 antagonists and proton-pump inhibitors) actually promote ibandronate absorption. Like the others in this therapeutic class, ibandronate is not metabolized, and that which is not bound to the bone (40–50% of the absorbed dose) is eliminated renally unchanged. It does not inhibit the cytochrome P450 (CYP450) isozymes. This agent does not require any dosage adjustment for patients with hepatic impairment or mild to moderate renal impairment (creatinine clearance, >30 mL/min). Ibandronate should not be prescribed for patients with severe renal impairment (creatinine clearance, <30 mL/min).Ибандронат натрия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +8618530059196 | China | 11805 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| 86-571-88216897,88216896 13588875226 |
CHINA | 6312 | 58 | |
| +862156762820 +86-13564624040 |
China | 7707 | 58 | |
| +8615255079626 | China | 23541 | 58 |