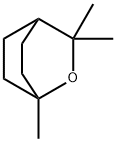
1,8-цинеол
- английское имя1,8-Cineole
- CAS №470-82-6
- CBNumberCB2853653
- ФормулаC10H18O
- мольный вес154.25
- EINECS207-431-5
- номер MDLMFCD00167977
- файл Mol470-82-6.mol
| Температура плавления | 1-2 °C(lit.) |
| Температура кипения | 176-177 °C(lit.) |
| плотность | 0.9225 |
| давление пара | 1.22hPa at 20℃ |
| FEMA | 2465 | EUCALYPTOL |
| показатель преломления | n |
| Fp | 122 °F |
| температура хранения | 2-8°C |
| растворимость | 3.5g/l |
| форма | Liquid |
| цвет | Clear colorless to slightly yellow |
| Запах | at 10.00 % in dipropylene glycol. eucalyptus herbal camphor medicinal |
| Odor Type | herbal |
| Вязкость | 3.4mm2/s |
| Растворимость в воде | Soluble in water(3500 mg/L (at 21°C). Miscible with ether, alcohol, chloroform, glacial acetic acid, oils. Soluble in ethanol, ethyl ether; slightly soluble in carbon tetrachloride. |
| Точка замерзания | min. 1.4 ℃ |
| Номер JECFA | 1234 |
| Мерк | 14,3895 |
| БРН | 105109 |
| Диэлектрическая постоянная | 4.8399999999999999 |
| Стабильность | Stable. Flammable. Incompatible with acids, bases, strong oxidizing agents. |
| ИнЧИКей | WEEGYLXZBRQIMU-WAAGHKOSSA-N |
| LogP | 3.4 |
| Справочник по базе данных CAS | 470-82-6(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | EUCALYPTOL |
| FDA 21 CFR | 310.545 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | RV6J6604TK |
| Код УВД | R05CA13 |
| Справочник по химии NIST | Eucalyptol(470-82-6) |
| Система регистрации веществ EPA | Eucalyptol (470-82-6) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi,F | |||||||||
| Заявления о рисках | 10-37/38-41-36/37/38 | |||||||||
| Заявления о безопасности | 26-39-16 | |||||||||
| РИДАДР | UN 1993 3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | OS9275000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2932 99 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 470-82-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2480 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
1,8-цинеол химические свойства, назначение, производство
Химические свойства
1,8-Cineole occurs in many terpene-containing essential oils, sometimes as the main component. For example, eucalyptus oils contain up to 85% 1,8-cineole and laurel leaf oil contains up to 70%. It is a colorless liquid with a characteristic odor, slightly reminiscent of camphor. 1,8-Cineole is one of the few fragrance materials that is obtained exclusively by isolation from essential oils, especially eucalyptus oils. Technical-grade 1,8- cineole with a purity of 99.6–99.8% is produced in large quantities by fractional distillation of Eucalyptus globulus oil. A product essentially free from other products can be obtained by crystallization of cineole-rich eucalyptus oil fractionsВхождение
Its name is derived from its presence in the essential oils of Eucalyptus globulus and Melaleuca leucadendron L. (essential oil of cajeput). It was originally identified in the essential oil of Artemisia maritime and subsequently in a large number (approx. 270) of other essential oils: rosemary, laurel leaves, clary sage, myrrh, cardamom, star anise, camphor, lavender, peppermint, Litsea guatemalensis, Luvunga scadens Roxb., Achillea micrantha and Salvia triloba. The essential oil of Eucalyptus polibrac tea has been reported to contain up to 91% eucalyptol. Also reported found in citrus oils and juices, guava, papaya, cinnamon bark, root and leaf, ginger, corn mint oil, spearmint, nutmeg, pepper, Thymus zygis, cardamom, cranberry, laurel, pepper, sweet marjoram, coriander, Spanish origanum, Ocimum basilicum, curcuma, sage, laurel, sweet and bitter fennel, myrtle leaf and berry, pimento and calamus.Использование
1,8-Cineol is the chief constituent of oil of eucalyptus. Used as pharmaceutic aid (flavor).Подготовка
By fractional distillation (170 to 180°C) from those essential oils containing high levels of eucalyptol, such as Eucalyptus globulus (approx. 60%), and subsequent separation of the product by congealing the distillate.Общее описание
Colorless liquid with a camphor-like odor. Spicy cooling taste.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
Cineole will react with acids and bases.Пожароопасность
Flash point data for Cineole are not available but Cineole is probably combustible.разработк И противоопухолевых препаратов
A statistically significant reduction of cell proliferation was observed compared tothe control cells when tested on RKO cells and human colon cancer cell linesHCT116 injected into the SCID mice. The 1,8-cineole induced apoptosis by inactivatingsurviving and Akt, activating p38, inducing PARP, and cleaving caspase-3(Rodd et al. 2015).Токсикология
Eucalyptol, a colourless organic compound, is a monoterpenoid and an ether. Among its various uses, eucalyptol is predominantly used as an insecticide and in fragrances (Klocke et al. 1987). The ques- tion of whether eucalyptol could potentiate toxicity has been assessed because of its widespread use in households. When Kunming mice received feed with a high dose of eucalyptol, liver and kidney tissue demonstrated vacuolar and granular degeneration (Xu et al. 2014). When the animals were fed with a subacute dose of eucalyptol, no discernible difference in body weight was observed (Xu et al. 2014). Eucalyptol’s safety profile has been assessed. Fatality after ingestion of eucalyptol oil has been reported; the approximate lethal dose of eucalyptol in the human is between 0.05 and 0.5 mL/kg of body weight (Hindle 1994). When male rats received eucalyptol, eosinophilic protein droplets accumulated in a dose- dependent fashion (Kristiansen and Madsen 1995). However, in a study of mutagenicity using CHO cells, eucalyptol did not induce mutagenicity as evidenced in the sister chromatid exchange assay (Galloway et al. 1987). Likewise, eucalyptol did not induce carcinogenicity in pathogen-free CFLP mice (Roe et al. 1979).Метаболизм
Eucalyptol undergoes oxidation in vivo with the formation of hydroxy cineole, which is excreted as hydroxycineoleglucuronic acid (Williams, 1959).Растворимость в органика
Soluble in Propylene glycol, miscible with alcohol and oils.Методы очистки
Purify 1,8-cineol by dilution with an equal volume of pet ether, then saturate with dry HBr. The precipitate is filtered off, washed with small volumes of pet ether, then cineole is regenerated by stirring the crystals with H2O. It can also be purified via its o-cresol or resorcinol addition compounds. Store it over Na until required. Purify it also by fractional distillation. It is insoluble in H2O but soluble in organic solvents. [IR: Kome et al. Nippon Kagaku Zasshi [J Chem Soc Japan (Pure Chem Sect)] 80 66 1959, Chem Abstr 603 1961, Beilstein 17 II 32, 17/1 V 273.]1,8-цинеол запасные части и сырье
сырьё
1of3
запасной предмет
1,8-цинеол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 028-87075086 13350802083 |
CHINA | 1824 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 |