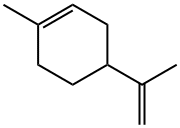
DL-Limonene
- русский язык имя
- английское имяDL-Limonene
- CAS №138-86-3
- CBNumberCB2178358
- ФормулаC10H16
- мольный вес136.23
- EINECS205-341-0
- номер MDLMFCD00001558
- файл Mol138-86-3.mol
| Температура плавления | -84--104 °C |
| Температура кипения | 170-180 °C (lit.) |
| плотность | 0.86 g/mL at 20 °C (lit.) |
| плотность пара | 4.7 (vs air) |
| давление пара | <3 mm Hg ( 14.4 °C) |
| показатель преломления | n |
| Fp | 119 °F |
| температура хранения | Store below +30°C. |
| растворимость | Chloroform: Slightly Soluble |
| форма | Liquid |
| цвет | Clear colorless to pale yellow |
| Запах | Pleasant, pine-like; lemon-like. |
| Пределы взрываемости | 0.7-6.1%, 150°F |
| Порог?обнаружения?запаха? | 0.038ppm |
| Odor Type | citrus |
| Биологические источники | synthetic |
| Растворимость в воде | <1 g/100mL |
| Мерк | 14,5493 |
| БРН | 3587825 |
| Диэлектрическая постоянная | 2.3(20℃) |
| Стабильность | Stable. Flammable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | AJSJXSBFZDIRIS-UHFFFAOYSA-N |
| LogP | 4.57 |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | DIPENTENE |
| FDA 21 CFR | 182.60 |
| Справочник по базе данных CAS | 138-86-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 9MC3I34447 |
| Словарь наркотиков NCI | limonene, (+)- |
| Справочник по химии NIST | Limonene(138-86-3) |
| Система регистрации веществ EPA | Limonene (138-86-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi,N | |||||||||
| Заявления о рисках | 10-38-43-50/53 | |||||||||
| Заявления о безопасности | 24-37-60-61 | |||||||||
| РИДАДР | UN 2052 3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | OS8350000 | |||||||||
| F | 8-10-23 | |||||||||
| Температура самовоспламенения | 458 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29021990 | |||||||||
| Банк данных об опасных веществах | 138-86-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 5300 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
DL-Limonene химические свойства, назначение, производство
Описание
D-limonene, which is a volatile oil, constitutes approximately 98% of orange peel oil by weight and has moderately good knockdown activity against ectoparasites of companion animals. The insecticidal activity of both d-limonene and linalool is enhanced when synergized by piperonyl butoxide. Apart from toxicoses reported in cats (65), d-limonene generally has a high margin of safety.Химические свойства
d-, l- or dl-Limonene has a pleasant, lemon-like odor free from camphoraceous and turpentine-like notes. Limonene is the most important and widespread terpene; it is known in the d- and l- optically active forms and in the optically inactive dl-form (known as dipentene).Вхождение
It has been reported found in more than 300 essential oils in amounts ranging from 90 to 95% (lemon, orange, mandarin) to as low as 1% (palmarosa); the most widespread form is the d-limonene, followed by the racemic form and then l-limo nene. Also reported found in ginger, nutmeg, pepper, mace, hop oil, coriander seed, calamus, dill herb, caraway seed and rosemary.Токсикология
D-limonene is a clear colorless mobile liquid with a pleasant lemon-like odor. ((4R)-limonene is an optically active form of limonene having (4R)-configuration. It has a role as a plant metabolite. It is an enantiomer of a (4S)-limonene.)D-limonene is one of the most common terpenes in nature. It is a major constituent in several citrus oils (orange, lemon, mandarin, lime, and grapefruit). D-limonene is listed in the Code of Federal Regulations as generally recognized as safe (GRAS) for a flavoring agent and can be found in common food items such as fruit juices, soft drinks, baked goods, ice cream, and pudding.Использование
d-Limonene is a flavoring agent that is a liquid, colorless with a pleasant odor resembling mild citrus. It is miscible in alcohol, most fixed oils, and mineral oil; soluble in glycerin; and insoluble in water and propylene glycol. It is obtained from citrus oil. It is also termed d-p-mentha-1,8,diene and cinene.Определение
ChEBI: A monoterpene that is cyclohex-1-ene substituted by a methyl group at position 1 and a prop-1-en-2-yl group at position 4 respectively.Подготовка
d-Limonene may be obtained by steam distillation of citrus peels and pulp resulting from the production of juice and cold pressed oils, or from deterpenation of citrus oils; it is sometimes redistilled.Методы производства
Limonene occurs in the oil of many plants and is the main constituent (≤86%) of the terpenoid fraction of fruit, flowers, leaves, bark, and pulp from shrubs, annuals, or trees including anise, mint, caraway, polystachya, pine, lime, and orange oil. It occurs as a by-product in the manufacture of terpineol and in various synthetic products made from α-pinene or turpentine oil. It is found in the gas phase of tobacco smoke and has been detected in urban atmospheres.Общее описание
A colorless liquid with an odor of lemon. Flash point 113°F. Density about 7.2 lb /gal and insoluble in water. Hence floats on water. Vapors heavier than air. Used as a solvent for rosin, waxes, rubber; as a dispersing agent for oils, resins, paints, lacquers, varnishes, and in floor waxes and furniture polishes.Реакции воздуха и воды
Flammable. Insoluble in water.Профиль реактивности
Cinene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas.Угроза здоровью
Liquid irritates eyes; prolonged contact with skin causes irritation. Ingestion causes irritation of gastrointestinal tract.Пожароопасность
Behavior in Fire: Containers may explode.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Контактные аллергены
Dipentene corresponds to a racemic mixture of d-limonene and l-limonene. Dipentene can be prepared from wood turpentine or by synthesis. It is used as a solvent for waxes, rosin and gums, in printing inks, perfumes, rubber compounds, paints, enamels, and lacquers. An irritant and sensitizer, dipentene caused contact dermatitis mainly in painters, polishers, and varnishersразработк И противоопухолевых препаратов
Tested as promising antitumor molecules in induced tumor on rat tissues, D-limonenewas tested in preclinical studies in patients with advanced cancer. Limonene inhibitsthe activity of HMG-CoA reductase, subsequently reducing the possibility of cancergrowth. The mechanism of action involves the inhibition of prenyltransferases withthe activation of glutathione-S transferase and uridine diphospho-glucuronosyltransferase.More interest was pointed on the principal metabolite:perillyl alcohol which is more potent than limonene. The interest on perillyl alcoholis based on the necessity of a very high dosage of D-limonene in preclinical studies(about 1000 mg/kg/day in human mammary tumor) that can cause notably importantside effects. The more active perillyl alcohol and the less low active doseshypothesized this molecule as a clinical candidate (Pattanayak et al. 2009; Chenet al. 2013; Fontes et al. 2013; Rani and Sharma 2013).Профиль безопасности
A skin irritant. Flammable when exposed to heat or flame; can react vigorously with oxidzing materials. When heated to decomposition it emits acrid smoke and irritating fumes.Канцерогенность
Induction of kidney neoplasias has been observed in male rats of strains that have significant concentrations of the protein a2u-globulin (158a). This protein is not expressed in females or species other than the rat; therefore, limonene carcinogenicity appears to be limited to the male of specific strains of this species. Subcutaneous injection of the compound or its hydroperoxide into C57BL/6 mice decreased the incidence of dibenzopyrene- induced tumors appreciably. Given orally either 15 min or 1 h prior to nitrosodiethylamine, D-limonene reduced forestomach tumor formation by about 60% and pulmonary adenoma formation by about 35%. Reduction of cancer incidence and metastasis by limonene has also been reported in other systems (158b).Экологическая судьба
Limonene is insoluble and is stable in water. Substances like limonene that are monoterpenes are released in large amounts mainly to the atmosphere. The chemical and physical properties of limonene also indicate that limonene is distributed mainly to air. Based on the physical and chemical properties of limonene, when this substance is released to ground, it has low to very low mobility in soil. The soil adsorption coefficient (Koc), calculated on the basis of the solubility (13.8 mg l-1 at 25 ℃) and the log octanol/water partition coefficient (4.232), ranges from 1030 to 4780.3. Henry’s Law constant indicates that limonene rapidly volatilizes from both dry and moist soil; however, its strong adsorption to soil may slow this process. In the aquatic environment, limonene is expected to adsorb to sediment and suspended organic particles to rapidly volatilize to the atmosphere, based on its physical and chemical properties. The estimated half-life for volatilization of limonene from a model river (1 m deep, flow 1 ms-1, and wind speed 3 ms-1) is 3.4 h. The bioconcentration factor, calculated on the basis of water solubility and the log octanol/water partition coefficient, is 246–262, suggesting that limonene may accumulate in fish and other aquatic organisms.DL-Limonene запасные части и сырье
сырьё
1of2
запасной предмет
1of2
DL-Limonene поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617653113219 | China | 2737 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 |