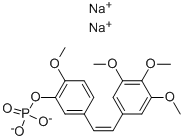
Combretastatin A4 phosphate disodium salt
- русский язык имя
- английское имяCombretastatin A4 phosphate disodium salt
- CAS №168555-66-6
- CBNumberCB2496827
- ФормулаC18H19O8P.2Na
- мольный вес440.29
- EINECS1312995-182-4
- номер MDLMFCD00943911
- файл Mol168555-66-6.mol
химическое свойство
| Температура плавления | 238-242 ºC |
| температура хранения | -20°C |
| растворимость | H2O: soluble5mg/mL, clear |
| форма | powder |
| цвет | white to beige |
| Растворимость в воде | H2O: 5mg/mL, clear |
| ИнЧИКей | VXNQMUVMEIGUJW-XNOMRPDFSA-L |
| Справочник по базе данных CAS | 168555-66-6 |
| FDA UNII | 702RHR475O |
| Словарь онкологических терминов NCI | combretastatin A4 phosphate |
| Словарь наркотиков NCI | fosbretabulin disodium |
| UNSPSC Code | 12352200 |
Combretastatin A4 phosphate disodium salt химические свойства, назначение, производство
Описание
Combretastatin A4 phosphate disodium salt, also known as fosbretabulin disodium or CA4P, is a microtubule destabilizing drug, a water-soluble prodrug of combretasain a4(ca4) and a tumor vascular-targeting agent.microtubule is the cylindrical and filamentous structure that required for cell shape, migration, cilia and flagella mobility etc. tubulin is the major component of microtubulesin proliferative endothelial cells, ca4p (dose less than 1/10 of the max. tolerated dose) leaded to tumor vascular shutdown, and short drug exposure of ca4p caused significant long-term anti-proliferative and cytotoxic effects. [1] in ca40 treated endothelia cell, myosin light chain was phosphorylated and lead to actinomyosin contractility, assembly of actin stress fibers and focal adhesions formation. [2] in huvec cells, ca4p blocked growth factor–induced endothelial cell proliferation and migration and impaired capillary tube formation. [3]in c57bl/6 mice, ca4p treatment in combination with mab against ve-cardherin facilitated b16 melanoma tumor necrosis and inhibited tumor neoangionesis. [3] in human breast cancer models in vivo, 6 hours after ca4p administration showed a 93% decreased functional vascular volume and lasted over the next 12 hours. [1]Химические свойства
White SolidПодготовка
Combretastatin A-4 phosphate can be synthesized by phosphorylation of combretastatin A-4 (I) following several related strategies. Direct phosphorylation of (I) employing either bis(2,2,2-trichloroethyl) phosphorochloridate in pyridine (1,2) or dibenzyl phosphite in the presence of CCl4 and DMAP (3-5) furnishes the corresponding bis-trichloroethyl (IIa) and dibenzyl (IIb) phosphate esters, which are subsequently deprotected to combretastatin A-4 phosphate (III) by either reductive trichloroethyl group cleavage with zinc dust and acetic acid (1,2) or by debenzylation with chlorotrimethylsilane/sodium iodide (3-5). Alternatively, phosphitylation of (I) using di-tert-butyl N,N- diethylphosphoramidite (3,4,6) or bis(trimethylsilylethyl) N,N-diisopropylphosphoramidite (3-5) in the presence of tetrazole yields the phosphite esters (IVa) and (IVb), respectively, which are further oxidized to the corresponding phosphates (Va) and (Vb) using m-chloroperbenzoic acid in CH2Cl2/THF (3-6). Combretastatin A-4 phosphate (III) is then obtained by acidic cleavage of the tert-butyl ester (Va) with trifluoroacetic acid (3,4,6) or trifluoromethanesulfonic acid (6), or by tetrabutylammonium fluoride-promoted cleavage of the trimethylsilylethyl ester (Vb) (3-5).Биохимия/физиол Действия
Fosbretabulin disodium (Combretastatin A4 disodium phosphate, CA4P) is a potent vascular-disrupting agent (VDA). Fosbretabulin disodium is a prodrug that is converted to combretastatin A inside the endothelial cells that line blood vessels, where it binds to tubulin dimers and prevents microtubule polymerization, resulting in mitotic arrest and apoptosis in endothelial cells. Fosbretabulin disodium is a potent anti-cancer agent that exhibits antivascular effects on tumor vasculature, inducing a rapid reduction in tumor blood flow and a concomitant increase of cellular necrosis. Some of its activity is believed to involve interference with vascular endothelial-cadherin signaling in addition to its microtubule-disrupting activity.Способ действия
Combretastatin A4 disodium phosphate works by quickly interrupting the blood supply to tumors, resulting in tumor death due to lack of oxygen and nutrients. Its mechanism of action in treating tumors is distinct from general cytotoxic chemotherapy drugs and drugs that inhibit the formation of new blood vessels. Unlike these drugs, Compradine Disodium Phosphate targets and breaks down the immature endothelial cells of newly formed blood vessels during tumor growth, while having no effect on the mature endothelial cells of normal tissue blood vessels.использованная литература
[1] dark gg, hill sa, prise ve, tozer gm, pettit gr, chaplin dj. combretastatin a-4, an agent that displays potent and selective toxicity toward tumor vasculature. cancer res. 1997 may 15;57(10):1829-34.[2] KANTHOU C, TOZER G M. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells.[J]. Blood, 2002, 99 6: 2060-2069. DOI:10.1182/BLOOD.V99.6.2060.
[3] VINCENT L, KERMANI P, YOUNG L M, et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling.[J]. The Journal of clinical investigation, 2005, 115 11: 2992-3006. DOI:10.1172/JCI24586.
[4] PRABHU S, HARRIS F, LEA R W, et al. Small-molecule clinical trial candidates for the treatment of glioma.[J]. Drug discovery today, 2014, 19 9: 1298-1308. DOI:10.1016/j.drudis.2014.02.007.
[5] TOCHINAI R, NAGATA Y, ANDO M, et al. Combretastatin A4 disodium phosphate-induced myocardial injury[J]. Journal of Toxicologic Pathology, 2016, 29: 163-171. DOI:10.1293/tox.2016-0012.
[6] PATTERSON D M, RUSTIN G J S, SERRADELL N, et al. Combretastatin A-4 phosphate[J]. Drugs of The Future, 2007, 32: 1025. DOI:10.1358/DOF.2007.032.12.1158960.
Combretastatin A4 phosphate disodium salt запасные части и сырье
Combretastatin A4 phosphate disodium salt поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617531153977 | China | 5855 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +8618957127338 | China | 2136 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |