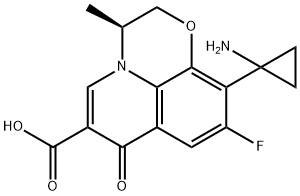
Пазуфлоксацин
- английское имяPazufloxacin
- CAS №127045-41-4
- CBNumberCB2423188
- ФормулаC16H15FN2O4
- мольный вес318.3
- EINECS635-036-1
- номер MDLMFCD00865012
- файл Mol127045-41-4.mol
химическое свойство
| Температура плавления | 269-271°C |
| альфа | D25 -88.0° (c = 0.5 in 0.05N aq NaOH) |
| Температура кипения | 531.5±50.0 °C(Predicted) |
| плотность | 1.56 |
| температура хранения | 2-8°C |
| растворимость | Aqueous Base (Slightly, Sonicated), DMSO (Slightly, Heated), Methanol (Slightly) |
| пка | 5.05±0.40(Predicted) |
| цвет | Off-White to Pale Yellow |
| Справочник по базе данных CAS | 127045-41-4(CAS DataBase Reference) |
| FDA UNII | 4CZ1R38NDI |
| Код УВД | J01MA18 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,C,F |
| Заявления о рисках | 20/21/22-34-11 |
| Заявления о безопасности | 36/37-45-36/37/39-26-16 |
| WGK Германия | 3 |
| RTECS | UU8815300 |
| Токсичность | LD50 i.v. in male mice: >500 mg/kg (Todo) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
Пазуфлоксацин химические свойства, назначение, производство
Описание
Pazufloxacin is a novel quinolone marketed for the treatment of bacterial infections in Japan. This tricyclic fluoro-quinolone can be synthesized in 11 steps from commercially available 2,3,4,5tetrafluorobenzoic acid. The cyclopropyl substituent is first introduced in 6 steps including 4-F-substitution with tert-butylcyanoacetate, decarboxylation, aa alkylation with 1 ,Zdibromoethane, partial nitrile hydrolysis and Hoffmann-rearrangement. The pyridoxazine ring is then introduced in 5 steps including 6-ketoester formation and pryridoxazine annulation. Pazufloxacin displays a broad spectrum activity against Grampositive and Gram-negative bacteria, although it is less active that ciprofloxacin against pneumococci and is not active against ciprofloxacin-resistant isolates. In patients with gonococcal urethritis a high prevalence of fluoroquinolone-resistant N. gonorrhoeae isolates with the Ser-91-to-Phe mutation in GyrA was observed. However, good clinical responses have been seen in clinical trials of patients with urinary tract infections and to a lesser extent with respiratory tract infections. Pazufloxacin is mainly excreted in urine with a short half-life (2-2.5 h). It has a phototoxicity equal to that of ciprofloxacin and its adverse effect profile resembles that of other quinolones.Использование
Pazufloxacin is a potential antimicrobial and/or antiviral agent.Фармацевтические приложения
A tricyclic fluoroquinolone, formulated as mesylate and hydrochloride salts for oral or parenteral use or as a methane sulfonate (eye ointment).It displays good activity in vitro against methicillin susceptible Staph. aureus (MIC 0.2 mg/L), but is inactive against Str. pyogenes, Str. pneumoniae (MIC ≥4 mg/L) and enterococci. L. pneumophila is inhibited by 0.03 mg/L. Activity against Enterobacteriaceae, fastidious Gram-negative bacilli, Ps. aeruginosa and Acinetobacter spp. is similar to that of ofloxacin. It is weakly active against Sten. maltophilia and Burkholderia cepacia (MIC c. 2 mg/L). Against M. tuberculosis, MICs range from 0.8 to 4 mg/L. It is inactive against anaerobes.
After oral doses of 100 or 400 mg, peak plasma concentrations range from 0.94 mg/L (100 mg) to 4.5 mg/L (400 mg) after <1 h. The apparent elimination half-life is around 2 h. Most of the administered dose is eliminated in urine, about 70% within 24 h. Four metabolites have been reported. In elderly patients, according to the renal function, the peak plasma concentration may be elevated (up to 5.6 mg/L) and significantly delayed (2–6 h). The plasma protein binding ranges from 17% to 28%.
Пазуфлоксацин запасные части и сырье
Пазуфлоксацин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617732866630 | China | 18147 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 |