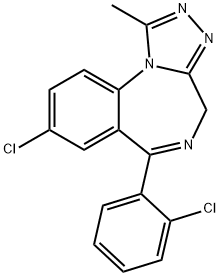
Триазолам
- английское имяTriazolam
- CAS №28911-01-5
- CBNumberCB2218405
- ФормулаC17H12Cl2N4
- мольный вес343.21
- EINECS249-307-3
- номер MDLMFCD00079623
- файл Mol28911-01-5.mol
| Температура плавления | 209-212°C |
| Температура кипения | 499.51°C (rough estimate) |
| плотность | 1.2835 (rough estimate) |
| показатель преломления | 1.6300 (estimate) |
| Fp | 11 °C |
| температура хранения | 2-8°C |
| растворимость | DMF: 30 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 20 mg/ml; Ethanol: 10 mg/ml |
| форма | A crystalline solid |
| пка | pKa 1.52(H2O) (Uncertain);6.5(H2O) (Uncertain) |
| Растворимость в воде | 30mg/L(ambient temperature) |
| Справочник по базе данных CAS | 28911-01-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 1HM943223R |
| Предложение 65 Список | Triazolam |
| Код УВД | N05CD05 |
| Справочник по химии NIST | Triazolam(28911-01-5) |
| Система регистрации веществ EPA | Triazolam (28911-01-5) |
| Коды опасности | F,T |
| Заявления о рисках | 11-23/24/25-36/38-39/23/24/25 |
| Заявления о безопасности | 22-24/25-26-36-45-36/37-16-7 |
| РИДАДР | 3249 |
| WGK Германия | 2 |
| RTECS | XZ5472500 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| кода HS | 2933910000 |
| Банк данных об опасных веществах | 28911-01-5(Hazardous Substances Data) |
| Токсичность | LD50 in mice, rats (mg/kg): >100, >5000 orally (Pharm. Weekblad.) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H370:Поражает органы (Глаза) в результате однократного воздействия.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P311:Обратиться за медицинской помощью.
Триазолам химические свойства, назначение, производство
Химические свойства
Yellow SolidИспользование
TriazolamВсемирная организация здравоохранения(ВОЗ)
Triazolam, a benzodiazepine derivative with sedative and hypnotic activity, was introduced in 1978 for themanagement of insomnia. It is controlled under Schedule IV of the 1971 Convention of Psychotropic Substances. Concern regarding the psychotropic effects of triazolam was first raised in the Netherlands in 1979 when this compound was suspended for sale and subsequently withdrawn by the Committee for the Evaluation of Medicines on the basis of reports of a reversible complex of symptoms including paranoia, depersonalization, nightmares, suicidal tendency and hyperaesthesia in patients receiving the drug. The basis for this decision was later successfully contested by the manufacturer and the drug was reregistered in early 1990 with a revised product information. However, concern was regenerated elsewhere that higher doses are associated with an unacceptable incidence of unwanted effects and the manufacturer has eventually withdrawn 0.5 mg tablets on a worldwide basis. In 1991 the issue of the safety of triazolam was again reopened by reports of retrograde amnesia and depression among patients taking the decreased recommended dosages. The product information has been revised by the United States FDA to include more rigorous cautions regarding dosage. In the Member States of the European Communities the products have been suspended pending further review by the EC Committee on Proprietary Medicinal Products.Общее описание
Triazolam, 8-chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo[4,3-a][1,4] benzodiazepine(Halcion), has all of the characteristic benzodiazepine pharmacologicalactions. It is an ultra–short-acting hypnoticbecause it is rapidly α-hydroxylated to the 1-methyl alcohol,which is then rapidly conjugated and excreted.Consequently, it has gained popularity as sleep inducers, especiallyin elderly patients, because it causes less daytimesedation. It is metabolically inactivated primarily by hepaticand intestinal CYP3A4; therefore, coadministration withgrapefruit juice increases its peak plasma concentration by30%, leading to increased drowsiness.Триазолам запасные части и сырье
сырьё