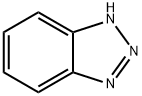
1H-бензотриазол
- английское имя1H-Benzotriazole
- CAS №95-14-7
- CBNumberCB4467102
- ФормулаC6H5N3
- мольный вес119.12
- EINECS202-394-1
- номер MDLMFCD00005699
- файл Mol95-14-7.mol
| Температура плавления | 97-99 °C(lit.) |
| Температура кипения | 204 °C (15 mmHg) |
| Плотность накопления | 500kg/m3 |
| плотность | 1,36 g/cm3 |
| плотность пара | 4.1 (vs air) |
| давление пара | 0.04 mm Hg ( 20 °C) |
| показатель преломления | 1.5589 (estimate) |
| Fp | 170 °C |
| температура хранения | Store below +30°C. |
| растворимость | 19g/l |
| форма | Powder, Granules, Crystals, Needles or Flakes |
| пка | 1.6(at 20℃) |
| цвет | White to yellow-beige |
| РН | 6.0-7.0 (100g/l, H2O, 20℃)suspension |
| Запах | Slight characteristic odor |
| Пределы взрываемости | 2% |
| Растворимость в воде | 25 g/l in water (20 ºC) |
| Мерк | 14,1108 |
| БРН | 112133 |
| Стабильность | Stable, but may be light sensitive. Incompatible with strong oxidizing agents, heavy metals. |
| ИнЧИКей | QRUDEWIWKLJBPS-UHFFFAOYSA-N |
| LogP | 1.34 at 22.7℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | BENZOTRIAZOLE |
| Справочник по базе данных CAS | 95-14-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 86110UXM5Y |
| Справочник по химии NIST | 1H-Benzotriazole(95-14-7) |
| Система регистрации веществ EPA | 1,2,3-Benzotriazole (95-14-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,Xi,F | |||||||||
| Заявления о рисках | 20/22-36-52/53-5-36/37/38-20/21/22-11 | |||||||||
| Заявления о безопасности | 26-36/37-61-45-36/37/39-28A | |||||||||
| РИДАДР | 2811 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DM1225000 | |||||||||
| Температура самовоспламенения | 400 °C | |||||||||
| Примечание об опасности | Harmful/Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29339990 | |||||||||
| Банк данных об опасных веществах | 95-14-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 560 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
1H-бензотриазол химические свойства, назначение, производство
Химические свойства
yellow to beige solid or Colorless needle-like crystals. Slightly soluble in cold water, ethanol and ether.Использование
1H-Benzotriazole is an anticorrosive agent, which is useful in aircraft deicing and antifreeze fluids. It is also employed in dishwasher detergents. Further, it is used as a restrainer in photographic emulsions and also useful as a reagent for the determination of silver in analytical chemistry. It also serves as a corrosion inhibitor in the atmosphere and underwater. Further, it is utilized in the synthesis of amines from glyoxal.Определение
ChEBI: 1H-Benzotriazole is the simplest member of the class of benzotriazoles that consists of a benzene nucleus fused to a 1H-1,2,3-triazole ring. It has a role as an environmental contaminant and a xenobiotic.Подготовка
1H-Benzotriazole is prepared by the reaction of o-phenylenediamine with nitrous acid in dilute sulfuric acid. Damschrodner and Peterson were able to synthesize the 1H-benzotriazole in a high yield (80%) by nitrosation of o-phenylenediamine with sodium nitrite in glacial acetic acid and water.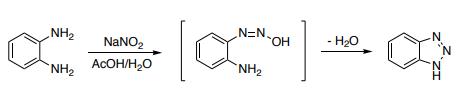
Synthesis of 1H-benzotriazole via diazotization of o-phenylenediamine
Reaction: Add o-phenylenediamine to 50°C water to dissolve, then add glacial acetic acid, cool down to 5°C, add sodium nitrite to stir the reaction. The reactant gradually turned dark green, the temperature rose to 70-80 ℃, the solution turned orange-red, placed at room temperature for 2 hours, cooled, filtered out the crystals, washed with ice water, dried to obtain the crude product, the crude product was distilled under reduced pressure, and collected 201 -204°C (2.0kPa) fraction, and then recrystallized with benzene to obtain 1H-Benzotriazole products with a melting point of 96-97°C, with a yield of about 80%.
прикладной
1H-Benzotriazole (BT) is a chemical used in a wide variety of industrial, commercial, and consumer products. It main used as an anticorrosive in metalworking, in art restoration, and as a tarnish remover and protective coating in the construction industry.In the aircraft industry, 1H-benzotriazole and tolyl benzotriazole are found to be the primary agents in most types of aircraft deicing/antiicing fluid (ADAFs).
Benzotriazole is also used as a component of aircraft deicing fluid, pickling inhibitor in boiler scale removal, restrainer, developer and antifogging agent in photographic emulsions, corrosion inhibitor for copper, chemical intermediate for dyes, in pharmaceuticals, and as fungicide. (HSDB 1998).
Benzotriazole(BTA), ethylenediamine tetraaceticacid(EDTA), and potassium iodide(KI) were used for preparing the polishing slurries.
Общее описание
White to light tan crystals or white powder. No odor.Реакции воздуха и воды
Dust may form an explosive mixture in air. Slightly soluble in water.Профиль реактивности
The triazoles are a group of highly explosive materials that are sensitive to heat, friction, and impact. Sensitivity varies with the type substitution to the triazole ring. Metal chelated and halogen substitution of the triazol ring make for a particularly heat sensitive material. Azido and nitro derivatives have been employed as high explosives. No matter the derivative these materials should be treated as explosives.Опасность
Highly toxic by ingestion. May explode under vacuum distillation.Угроза здоровью
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1H-Benzotriazole emits toxic fumes. 1H-Benzotriazole can react violently during vacuum distillation.Пожароопасность
Flash point data are not available for 1H-Benzotriazole. 1H-Benzotriazole is probably combustible.Профиль безопасности
Poison by intravenous route.Moderately toxic by ingestion and intraperitoneal routes.Questionable carcinogen with experimental tumorigenicdata. Mutation data reported. May detonate at 220°C or during vacuum distillation. When heated to decompositionitВозможный контакт
Because benzotriazoles are used in large quantities as a corrosion inhibitor, it is mainly through this type of use that benzotriazoles become an environmental contaminant. As a corrosion inhibitor and fire retardant, they are used in antifreeze in concentrations of 0.01-2.0% and in airplane deicing/antiicing fluids in unknown concentrations, up to 10% (Cancilla et al.,1997). Used antifreeze may leak or be poured down drains and thence enters the environment. Also, an estimated 80% of aircraft deicing/anti-icing fluids are deposited on the ground due to spray drift, jet blast, and wind shear during taxiing and takeoff, according to a recent study (Hartwell et al., 1995).Канцерогенность
Chronic (2-year) feeding studies were conducted. Rats were given 0, 6700, or 12,000 ppm in feed for 78 weeks and held for an additional 26 weeks. Mice were given 0, 11,700, or 23,500 ppm in feed in 104 weeks. The authors concluded that under the conditions shown in this study, there were no convincing evidence that 1-H-benzotriazole was carcinogenic in rats or mice.Методы очистки
1,2,3-Benzotriazole crystallises from toluene, CHCl3, Me2NCHO or a saturated aqueous solution, and is dried at room temperature or in a vacuum oven at 65o. Losses are less if the material is distilled in a vacuum. CAUTION: may EXPLODE during distillation; necessary precautions must be taken. [Damschroder & Peterson Org Synth Coll Vol III 106 1955, Beilstein 26 III/IV 93.]1H-бензотриазол запасные части и сырье
сырьё
1of3
запасной предмет
- 5-метил-1Н-бензотриазол
- Inhibitor
- Antirust grease
- new scale corrosion inhibitor W-331
- N-METHYL-O-TOLUIDINE
1of3
1H-бензотриазол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-315-8309571 +8615633399667 |
China | 574 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-16632316109 | China | 1085 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8615373025980 | China | 895 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 |
1H-бензотриазол Обзор)
1of3