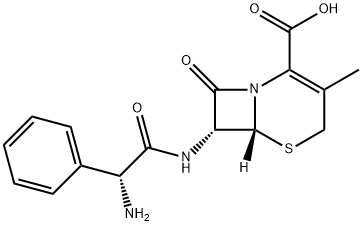
Cephalexin
- русский язык имя
- английское имяCephalexin
- CAS №15686-71-2
- CBNumberCB1210543
- ФормулаC16H17N3O4S
- мольный вес347.39
- EINECS239-773-6
- номер MDLMFCD04966776
- файл Mol15686-71-2.mol
| Температура плавления | 196-198°C |
| альфа | [α]D20 +144~+158° (c=0.5, H2O) (Calculated on dehydrous basis) |
| Температура кипения | 727.4±60.0 °C(Predicted) |
| плотность | 1.3040 (rough estimate) |
| показатель преломления | 1.6320 (estimate) |
| температура хранения | Keep in dark place,Inert atmosphere,2-8°C |
| растворимость | NH4OH 1 M: 50 mg/mL, clear, yellow |
| форма | Solid |
| пка | 5.2, 7.3(at 25℃) |
| цвет | White to light yellow |
| РН | pH (5g/l, 25℃) 3.5~5.5 |
| Растворимость в воде | 12.5g/L(25 ºC) |
| Мерк | 13,1986 |
| BCS Class | 4 |
| ИнЧИКей | AVGYWQBCYZHHPN-CYJZLJNKSA-N |
| Справочник по базе данных CAS | 15686-71-2(CAS DataBase Reference) |
| FDA UNII | 5SFF1W6677 |
| Словарь онкологических терминов NCI | cephalexin |
| Словарь наркотиков NCI | cephalexin |
| Код УВД | J01DB01 |
| Система регистрации веществ EPA | 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-, (6R,7R)- (15686-71-2) |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 42/43 | |||||||||
| Заявления о безопасности | 22-36/37-45 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | XI0350000 | |||||||||
| кода HS | 29419000 | |||||||||
| Банк данных об опасных веществах | 15686-71-2(Hazardous Substances Data) | |||||||||
| Токсичность | TDLo orl-hmn: 14 mg/kg/D:GIT AACHAX -,361,68 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
Cephalexin химические свойства, назначение, производство
Описание
Use of the ampicillin-type side chain conveys oral activity to cephalexin. Whereas it no longer has an activating side chain at C-3 and, as a consequence, is somewhat less potent, it does not undergo metabolic deactivation and, thus, maintains potency. It is rapidly and completely absorbed from the GI tract and has become quite popular. Somewhat puzzling is the fact that the use of the ampicillin side chain in the cephalosporins does not result in a comparable shift in antimicrobial spectrum. Cephalexin, like the other first-generation cephalosporins is active against many Gram-positive aerobic cocci but is limited against Gram-negative bacteria. It is a widely used drug, particularly against Gram-negative bacteria causing urinary tract infections, Gram-positive infections (Staphyl ococcus aureus, Streptococcus pneumoni ae and Streptococcus pyogenes) of soft tissues, pharyngitis, and minor wounds.Химические свойства
White cryst. powderИспользование
Antibacterial.Определение
ChEBI: A semisynthetic first-generation cephalosporin antibiotic having methyl and beta-(2R)-2-amino-2-phenylacetamido groups at the 3- and 7- of the cephem skeleton, respectively. It is effective against both Gram-negative and G am-positive organisms, and is used for treatment of infections of the skin, respiratory tract and urinary tract.Антимикробная активность
It is resistant to staphylococcal β-lactamase. Gram-positive rods and fastidious Gram-negative bacilli, such as Bordetella spp. and H. influenzae, are relatively resistant. It is active against a range of enterobacteria, but it is degraded by many enterobacterial β-lactamases. Citrobacter, Edwardsiella, Enterobacter, Hafnia, Providencia and Serratia spp. are all resistant. Gram-negative anaerobes other than B. fragilis are susceptible. Because of its mode of action it is only slowly bactericidal to Gram-negative bacilli.Фармакокине?тика
Oral absorption: >90%Cmax 500 mg oral: c. 10–20 mg/L after 1 h
Plasma half-life: 0.5–1 h
Volume of distribution: 15 L
Plasma protein binding: 10–15%
Absorption and distribution
It is almost completely absorbed when given by mouth, the peak concentration being delayed by food. Intramuscular preparations are not available: injection is painful and produces delayed peak plasma concentrations considerably lower than those obtained by oral administration.
In synovial fluid, levels of 6–38 mg/L have been described after a 4 g oral dose, but penetration into the CSF is poor. Useful levels are achieved in bone (9–44 mg/kg after 1 g orally) and in purulent sputum. Concentrations of 10–20 mg/L have been found in breast milk. Concentrations in cord blood following a maternal oral dose of 0.25 g were minimal.
Metabolism and excretion
It is not metabolized. Almost all the dose is recoverable from the urine within the first 6 h, producing urinary concentrations exceeding 1 g/L. The involvement of tubular secretion is indicated by the increased plasma peak concentration and reduced urinary excretion produced by probenecid. Renal clearance is around 200 mL/min and is depressed in renal failure, although a therapeutic concentration is still obtained in the urine. It is removed by peritoneal and hemodialysis. Some is excreted in the bile, in which therapeutic concentrations may be achieved.
Клиническое использование
Cephalexin, 7α-(D-amino-α-phenylacetamido)-3-methylcephemcarboxylicacid (Keflex, Keforal), was designed purposelyas an orally active, semisynthetic cephalosporin. Theoral inactivation of cephalosporins has been attributed to twocauses: instability of the β-lactam ring to acid hydrolysis(cephalothin and cephaloridine) and solvolysis or microbialtransformation of the 3-methylacetoxy group (cephalothin,cephaloglycin). The α-amino group of cephalexin renders itacid stable, and reduction of the 3-acetoxymethyl to a methylgroup circumvents reaction at that site.Cephalexin occurs as a white crystalline monohydrate. Itis freely soluble in water, resistant to acid, and absorbed wellorally. Food does not interfere with its absorption. Becauseof minimal protein binding and nearly exclusive renal excretion,cephalexin is recommended particularly for the treatmentof urinary tract infections. It is also sometimes used forupper respiratory tract infections. Its spectrum of activity isvery similar to those of cephalothin and cephaloridine.Cephalexin is somewhat less potent than these two agentsafter parenteral administration and, therefore, is inferior tothem for the treatment of serious systemic infections.
Побочные эффекты
Nausea, vomiting and abdominal discomfort are relatively common. Pseudomembranous colitis has been described and overgrowth of Candida with vaginitis may be troublesome. Otherwise, mild hypersensitivity reactions and biochemical changes common to cephalosporins occur. Very rare neurological disturbances have been described, particularly in patients in whom very high plasma levels have been achieved. There are rare reports of Stevens–Johnson syndrome and toxic epidermal necrolysis.Профиль безопасности
Poison by intraperitoneal route.Moderately toxic by ingestion and other routes. An experimental teratogen. Other experimental reproductiveeffects. Human systemic effects by ingestion: nausea,vomiting, and diarrhea. When heated to decomposition itemitsCephalexin запасные части и сырье
Cephalexin поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined18318671572 | China | 1000 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +8615102730682 | CHINA | 566 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18631714998 | CHINA | 904 | 58 |