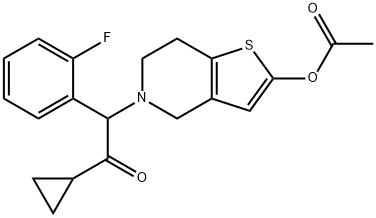
Прасугрел
- английское имяPrasugrel
- CAS №150322-43-3
- CBNumberCB01463424
- ФормулаC20H20FNO3S
- мольный вес373.44
- EINECS801-962-1
- номер MDLMFCD09954140
- файл Mol150322-43-3.mol
химическое свойство
| Температура плавления | 120.0 to 124.0 °C |
| Температура кипения | 493.5±45.0 °C(Predicted) |
| плотность | 1.347 |
| температура хранения | 2-8°C |
| растворимость | DMSO: >5mg/mL (warmed at 60 °C) |
| пка | 3.65±0.20(Predicted) |
| форма | powder |
| цвет | white to beige |
| ИнЧИКей | DTGLZDAWLRGWQN-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 150322-43-3(CAS DataBase Reference) |
| FDA UNII | 34K66TBT99 |
| Код УВД | B01AC22 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Заявления о безопасности | 24/25 |
| WGK Германия | 3 |
| кода HS | 29349990 |
| Банк данных об опасных веществах | 150322-43-3(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Прасугрел химические свойства, назначение, производство
Описание
Prasugrel is a third-generation thienopyridine that has been developed and launched for the prevention of atherothrombotic events in patients with ACS or following PCI. While the second-generation agent clopidogrel was an improvement over the first-generation ticlopidine, which suffered from gastrointestinal adverse effects and the risk of neutropenia with prolonged use, its delayed onset of action and considerable interpatient variability prompted the search for the next-generation thienopyridine. The mechanism of action of these platelet inhibitors involves initial biological activation to a sulfhydryl metabolite that irreversibly binds to the P2γ12 receptor on platelets via disulfide formation, thereby preventing platelet activation and aggregation by the endogenous agonist adenosine diphosphate (ADP). The advantage of prasugrel over its predecessors is its more efficient and consistent absorption and rapid conversion to its active metabolite. Co-administration of thienopyridines with acetylsalicylic acid (aspirin), an inhibitor of the synthesis of the platelet aggregation mediator thromboxane A2, is an effective antiplatelet strategy and joins antagonists of glycoprotein IIb/IIIa, which target the final step in platelet aggregation, in the medical arsenal combating atherothrombotic events.Использование
Prasugrel is a platelet inhibitor that reduces aggregation of platelets by being a P2Y12(ADP receptor) inhibitor.Клиническое использование
Prasugrel is a platelet inhibitor developed by Daiichi Sankyo Co. and is marketed in the United States in cooperation with Eli Lilly and Company for acute coronary syndromes planned for percutaneous coronary intervention (PCI). Prasugrel was approved for use in Europe in February 2009, and is currently available in the UK. In the U.S. prasugrel is also approved for the reduction of thrombotic cardiovascular events, including stent thrombosis, in patients with acute coronary syndrome who are to be managed with PCI. Prasugrel is a member of the thienopyridine class of ADP receptor inhibitors, and irreversibly binds to P2Y12 receptors.Побочные эффекты
In addition to the hemorrhagic side effect, other serious adverse events included AF, bradycardia, leucopenia, severe thrombocytopenia, angiodema, anemia, and abnormal hepatic function with hypertension, headache, back pain, dyspnea, nausea, dizziness, and diarrhea as less severe complaints. Prasugrel is contraindicated in patients with active pathological bleeding, such as peptic ulcers or intracranial hemorrhage, and in patients with a history of prior transient ischemic attack or stroke. In addition, in patients 75 years old, <60 kg, or likely to undergo urgent coronary artery bypass graft surgery, the risk may not outweigh the benefit. When possible, prasugrel treatment should be discontinued at least 7 days prior to any surgery. While warfarin and non-steroidal antiinflammatory drugs (NSAIDS) may increase the risk of bleeding with coadministration of prasugrel, no drug interactions are anticipated with concomitant use of drugs that are inducers or inhibitors of the cytochrome P450 enzymes. Prasugrel may also be administered with aspirin (75-325 mg per day), heparin, GP IIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including PPIs and H2 blockers.Прасугрел запасные части и сырье
сырьё
- Ethanone, 1-cyclopropyl-2-(2-fluorophenyl)-2-[(methylsulfonyl)oxy]-
- 2-бром-2- (2-фторфенил) -1-циклопропилетанон
- 5-[2-Cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2(3H)-one
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridin-2(4H)-one 4-methylbenzenesulfonate
- ТИЕНО [3,2-C] ПИРИДИН-2 (3H) -ОН, 4,5,6,7-ТЕТРАГИДРО-, ГИДРОХЛОРИД (1: 1)
1of3
Прасугрел поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-18031140164 +86-19933155420 |
China | 100 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +8617756083858 | China | 973 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| 15950718863 | CHINA | 295 | 58 |