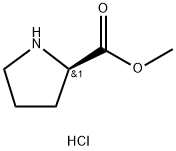
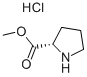
The general steps for the synthesis of L-proline methyl ester hydrochloride from methanol and DL-proline are as follows:
Step 1: Synthesis of methylpyrrolidine-2-carboxylic acid hydrochloride
Thionyl chloride (15.7 mL, 217.4 mmol) was slowly added dropwise to a mixed solution of DL-proline (5.0 g, 43.47 mmol) and methanol (50 mL) at about 0 °C, and the dropwise process lasted for about 15 minutes. The reaction mixture was stirred at room temperature for about 16 hours before the solvent was removed by vacuum concentration. The resulting gel was ground with n-pentane, decanted and dried to give D-proline methyl ester hydrochloride as an off-white solid (6.25 g, 87% yield).
The product characterization data were as follows:
1H NMR (400 MHz, DMSO-d6) δ 1.87-2.04 (m, 3H), 2.21-2.28 (m, 1H), 3.22-3.26 (m, 2H), 3.75 (s, 3H), 4.34 (t, J = 7.8 Hz, 1H).
IR (film) υ 3513, 3130, 2597, 1740, 1632, 1452, 1402, 1245, 1178, 1046, 764 cm-1;
MS 130 (M + 1).