Why is PCl3 a polar molecule?
PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole.
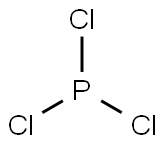
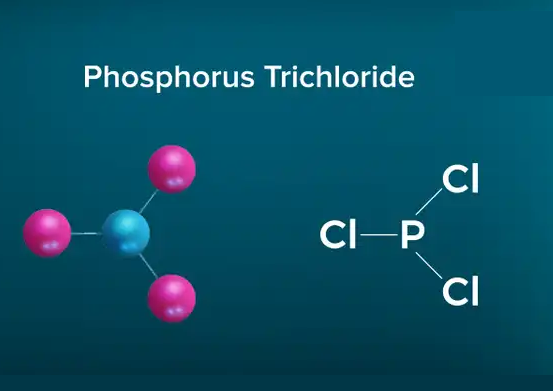
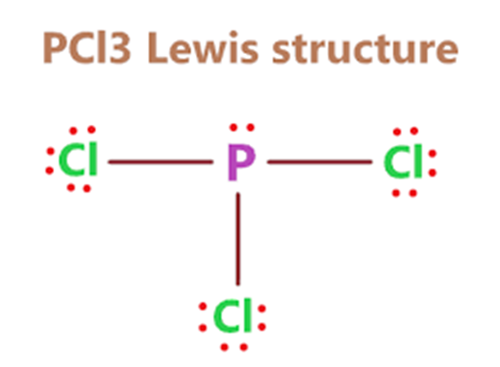
Due to the existence of one lone pair on the phosphorus atom, the Phosphorus trichloride(PCl3) molecule has a twisted trigonal pyramidal form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule.

The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. PCl3 has a dipole moment of 0.97D across the entire molecule. The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule.
Because of the asymmetric tetrahedral shape of the PCl3 molecule, the charge is dispersed non-uniformly among the phosphorus and three chlorine atoms, resulting in the formation of positive and negative poles across the PCl3 molecule.
So, it makes, PCl3 is a polar molecule.