Phosphorus trichloride: Chemical properties, uses and toxicity
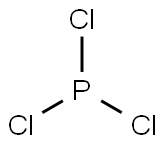
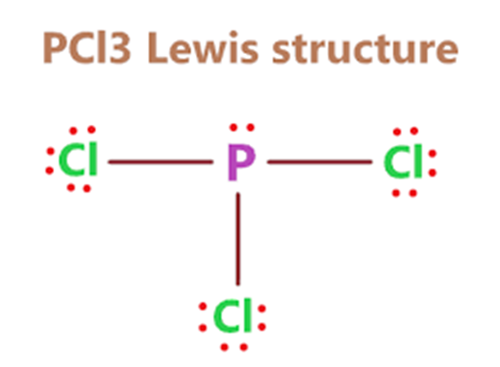
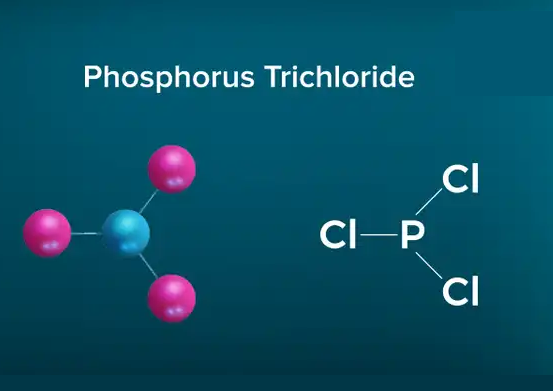
Phosphorus trichloride (PCl3) is a colorless or slightly yellowish fuming liquid with a hydrochloric acid odor. It is soluble in water, alcohol, benzene, chloroform and ether. PCl3 reacts violently with water to release phosphoric acid and hydrogen chloride (HCl) gas. It is easily decomposed by heating and is corrosive and explosive. PCl3 is also a strong oxidant and reacts easily with many organic compounds.
Uses
Phosphorus trichloride is an important chemical reagent or intermediate component that can be used for electrodeposition of metals on rubber, as well as for the manufacture of pesticides, surfactants, gasoline additives, plasticizers, dyes, textile finishing agents, fungicides, pharmaceutical products and other chemicals.
Application of PCl3 in synthesis
In the presence of 4-dimethylaminopyridine catalyst, amides and PCl3 are heated at 75~80°C for 1 hour to synthesize imidoyl chloride with a yield of 63-99%. PCl3 can be used as a raw material for the preparation of phosphorus (P)-containing polymers (PCP). PCP is widely used in biomedicine and structural materials due to its biodegradability and flame retardancy. However, the use of PCl3 to prepare PCP not only causes severe acid pollution and produces a large amount of chlorine-containing solid byproducts, but most importantly, there are safety risks. Therefore, the use of PCl3-free routes to prepare PCP is currently being developed. The sequential nucleophilic substitution of PCl3 with alcohols efficiently synthesizes asymmetric phosphotriesters containing acid- and/or base-labile functional groups.
Phosphorylation
Studies have shown that PCl3 can be used to prepare copper-chitosan phosphate complexes. Surface phosphorylation of chitosan (Cts) is achieved by exposing it to PCl3 vapor. The formed chitosan-dichlorophosphine (Cts-[Y-PCl2]z; Y = N,O; z ≤ 3) is hydrolyzed to chitosan-hydrogen phosphate (Cts-[Y-P(O)(H)(OH)]z). Antimicrobial testing of the synthesized Cts-[Y-P(O)(H)(OH)]z×Cu2+ against representative Gram-negative bacteria (Escherichia coli) and Gram-positive bacteria (Staphylococcus aureus) revealed their potential applications as antimicrobial materials.
Toxicity
Phosphorus trichloride is toxic to humans. It is a corrosive chemical that is a strong irritant to the eyes and skin, causing skin burns upon contact and possible eye damage. Inhalation may cause coughing and shortness of breath. Exposure to PCl3 has been reported to cause symptoms such as eye irritation, tearing, nausea, vomiting, and difficulty breathing.
References:
[1] E. V. SHISHKIN. Synthesis of Imidoyl Chlorides Using Phosphorus Trichloride[J]. Russian Journal of Organic Chemistry, 2021, 57 5. DOI:10.1134/S1070428021050134.[2] YU-LIN HONG . Progress in the preparation of phosphorus-containing polymers via phosphorus trichloride-free routes[J]. European Polymer Journal, 2023, 195. DOI:10.1016/j.eurpolymj.2023.112242.
[3] HIROSHI KITAMURA. Cover Feature: Sequential Nucleophilic Substitution of Phosphorus Trichloride with Alcohols in a Continuous-Flow Reactor and Consideration of a Mechanism for Reduced Over-reaction through the Addition of Imidazole (Chem. Eur. J. 37/2022)[J]. Chemistry - A European Journal, 2022, 28 37. DOI:10.1002/chem.202201646.
[4] MARCIN H. KUDZIN . Phosphorylation of chitosan (chitin) surface with PCl3[J]. Phosphorus, Sulfur, and Silicon and the Related Elements, 2022, 197 5: 393-659. DOI:10.1080/10426507.2021.2014489.