PCl3 Lewis structure: Drawing, Hybridisation, Geometry
PCl3 Lewis structure
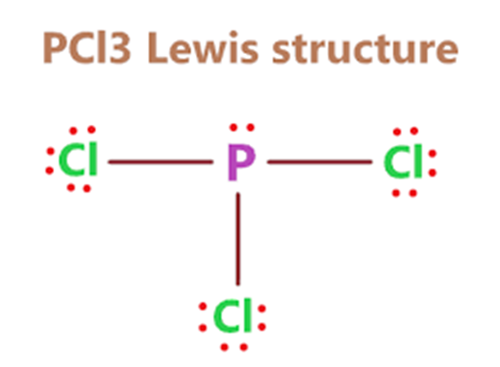
The Lewis structure of PCl3 consists of a central phosphorus atom (P) and three external chlorine atoms (Cl). There are three single bonds between the phosphorus atom (P) and each of the chlorine atoms (Cl). There is one lone pair of electrons on the phosphorus atom (P) and three lone pairs of electrons on each of the chlorine atoms (Cl).The PCl3 Lewis structure is shown below:
Steps for drawing the PCl3 Lewis structure
Step 1 Calculate the number of valence electrons for P and Cl
Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. Therefore, there are 5 and 7 valence electrons in the phosphorus and chlorine atoms respectively, so the total number of valence electrons in the PCl3 molecule = valence electrons from 1 phosphorus atom + valence electrons from 3 chlorine atoms = 5 + 7(3) = 26.
Step 2 Identify the central atom
The central atom must be highly or minimally electronegative. For the PCl3 molecule, the phosphorus atom is less electronegative, so the phosphorus (P) atom is the central atom and the chlorine (Cl) atom is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. For the PCl3 molecule, the total number of electron pairs is 14. The phosphorus atom is connected to each of the three chlorine atoms by a σ-bond (one σ-bond equals one pair of electrons), and the remaining 11 pairs of electrons are distributed as follows: one lone pair of electrons on the phosphorus atom, and three pairs of lone electrons on each of the chlorine atoms (3).
Step 4 Stability of structure
In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. The remaining one lone pair of electrons is on the central phosphorus atom, which also forms an octet of stable structure. Therefore, the Lewis structure of PCl3 in the above step is stable and has not changed further.
PCl3 Hybridisation
Phosphorus in PCl3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. These hybrid orbitals overlap with the p orbitals of chlorine atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PCl3.
Geometry of PCl3
The molecular shape of PCl3 is trigonal pyramidal. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape. The bond angles between the central phosphorus atom and the three chlorine atoms are approximately 109.5 degrees.