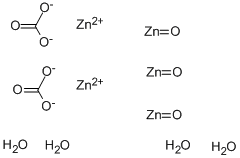
What is Zinc carbonate
Introduction
Zinc Carbonate (ZnCO3) is soluble in alkalis and acids. It is generally transparent and colourless in pure form, but it is coloured by the presence of iron, manganese, copper or other elements when it is not in pure form. Zinc carbonate is an important zinc source which can be easily converted to other zinc compounds, such as zinc oxide. This process can be achieved by heating, which results in zinc oxide and carbon dioxide formation. This process is also known as calcination. This reaction can also be achieved by reacting zinc carbonate with dilute acids. Notably, zinc carbonate, as an inorganic salt, is commonly used as a catalyst in organic synthesis reactions. Furthermore, this inorganic compound is an appropriate precursor for fabricating zinc oxide particles. It is used in pharmaceuticals to make other zinc compounds as a feed additive.

Is zinc carbonate acidic or basic?
As with other carbonates, zinc carbonate is readily dissolved in acidic solutions due to its basic nature but is insoluble in water. The reaction yields carbon dioxide. This will also dissolve to form zincates in an extremely strong base. The zinc carbonate can be decomposed thermally to form zinc oxide.
Is zinc carbonate toxic?
You can be affected by zinc carbonate when you breathe it in. The skin and eyes can get irritated by contact. Zinc carbonate can also irritate the nose and throat, causing coughing and wheezing. Elevated exposure can affect the liver.
You may like
Related articles And Qustion
See also
Lastest Price from Zinc carbonate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 3486-35-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 3486-35-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt