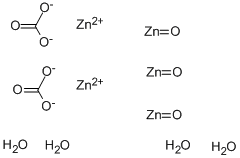
Application of Zinc carbonate
General description
Zinc carbonate appears as a white crystalline solid or powder that is insoluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Used in pharmaceuticals, to make other zinc compounds, as a feed additive. Zinc carbonate is the carbonate of zinc. The chemical formula is ZnCO3. It is a white fine amorphous powder and tasteless. The main component of smithsonite is formed in the oxidation zone of secondary mineral weathering or zinc bearing deposit. Sometimes it replaces carbonate rock mass and may form zinc ore. Zinc carbonate is used as light astringent, preparation of calamine, skin protector and raw material of latex products.
Basic zinc carbonate is a white fine amorphous powder, odorless, tasteless, insoluble in water and alcohol, slightly soluble in ammonia, soluble in dilute acid and sodium hydroxide, and 30% H202, release C02 and form peroxide. When heated at 250 ~ 500 ℃ for different times and cooled to room temperature, fluorescence phenomenon can occur. Its molecular formula is ~ indefinite formula. Under the condition of X-ray diffraction.
Application and pharmacology
Zinc carbonate is used as skin protector in medicine, zinc supplement in feed, light astringent and latex products in industry, and calamine lotion. It can also be used as the main raw material of desulfurizer and catalyst in rayon and chemical fertilizer industry. It can also be widely used in rubber products, oil paint and other chemical products, and in oil drilling, This product can react with H2S to form stable insoluble ZnS, and the product will not affect the mud performance after adding mud. Therefore, it can effectively eliminate the pollution and corrosion of H2S. It can be used as a corrosion inhibitor and sulfur remover for oil and gas wells containing H2S. Used in EVA foaming industry, it plays the role of uniform foaming and alleviating AC / ADC foaming agent. It is widely used as desulfurizer in medicine, rubber, latex products, petrochemical industry and chemical fertilizer.[1]
Synthesis
Zinc carbonate hydroxide was synthesized by the homogeneous precipitation method from urea and zinc nitrate, The influence of reaction duration on the utilization of Zn atom from solution into precipitate was investigated under different temperature, molar ratio of Zn to urea. XRD and SEM were used to characterize the crystal phase and morphology of the precipitates, respectively. During the first 20 minutes when the solution was heated from room temperature to about 85 ℃,the pH value firstly decreased and then increased. ZnO crystal was detected in the precipitates formed during the first 20 minutes, but only Zn CO(OH)·HO(Zn4) and Zn(CO:)(OH);(Zn5) were found in the precipitates with extended reaction time longer than 40 minutes. The partial transformation of Zn4 into Zn5 was found according to the XRD results, which result in a slowly decreased pH value after 30 minutes in the reaction. Zine carbonate hvdroxides appear as sphere particles assembled bv plate like unit. After calcination. ZnO crystal was formed which maintained the same morphology as zinc carbonate hydroxides with much pores in the plate like unit. The size of ZnO crystal raised with the increase of calcination temperature.[2,3]
Figure the systhesis route of Zinc carbonate
Toxicity
Inhalation: if inhaled, move the patient to fresh air. Skin contact: take off contaminated clothes and wash skin thoroughly with soapy water and clear water. If you feel unwell, see a doctor. Eye contact: separate eyelids and rinse with flowing water or normal saline. Seek medical attention immediately. Ingestion: gargle and do not induce vomiting. Seek medical attention immediately. Advice to protect the rescuer: transfer the patient to a safe place. Consult a doctor. Show this chemical safety technical manual to the doctor on site. Special tips for doctors: no data.
Storage
Packed in plastic woven bags lined with polyethylene plastic bags, with a net weight of 25kg each. Store in a cool, ventilated and dry warehouse. It shall not be stored and transported together with acid-base items. Pay attention to moisture. During transportation, it shall be protected from rain, moisture, sun and heat. In case of fire, water can be used for rescue. Sand and fire extinguisher.
Reference
1.Preparation of basic zinc carbonate by homogeneous precipitation
2.Bao-ping Z., Jin-long Z. & Mo-tang T. et al., "Thermodynamic Analysis of Carbonation Process and Characterization of Basic Zinc Carbonate," HYDROMETALLURGY OF CHINA, Vol.24, No.4(2005), p.199-202.]
3.Preparation of nano microcrystalline basic zinc carbonate
You may like
Related articles And Qustion
Lastest Price from Zinc carbonate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 3486-35-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 3486-35-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt