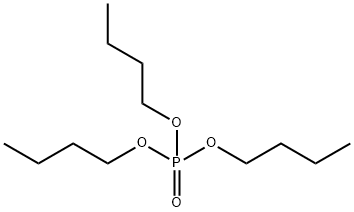
What is Tributyl phosphate?
Tributyl phosphate (TBP, C12H27O4P, CAS registry No. 126-73-8) is an odorless colorless to yellow liquid. Its melting point is -79 oC, and boiling point is 289 oC. The solubility of TBP is only 280 mg/L in water at 25 oC. It is soluble in diethyl ether, benzene, carbon disulfide. It can be miscible with ethanol. It is stable, but it is incompatible with strong oxidizing agents. So, store in a cool, dry place. Keep from contact with oxidizing materials. Keep away from reducing agents. Keep containers tightly closed.
TBP is a trialkyl phosphate that is the tributyl ester of phosphoric acid. TBP is a toxic organophosphorous compound widely used in many industrial applications, including significant usage in nuclear processing. TBP is a solvent and plasticizer for cellulose esters such as nitrocellulose and cellulose acetate. The major uses of TBP in industry are as a component of aircraft hydraulic fluid and as a solvent for extraction and purification of rare earth metals from their ores, such as uranium and plutonium. TBP is used also in mercerizing liquids, where it improves their wetting properties. TBP is also used as a heat exchange medium. TBP is used in some consumer products such as herbicides and water thinned paints and tinting bases.
The industrial application of this chemical is responsible for occupational exposure and environmental pollution. Exposure to TBP can be from ingestion, inhalation, or skin or eye contact. This exposure will most often happen from occupational use of hydraulic fluid. If TBP is released to the environment, it will bind tightly to dust particles in the air. Unbound TBP will break down in air. It will move slowly through soil because it will bind with soil particles. It may volatilize slowly from moist soil and water surfaces. It may build up in aquatic organisms. It will be broken down in water by microbes.
1H NMR-based metabonomics has been applied to investigate the metabolic response to TBP exposure[1]. Male Sprague-Dawley rats were given a TBP-dose of 15 mg/kg body weight, followed by 24 h urine collection, as was previously demonstrated for finding most of the intermediates of TBP. High-resolution 1H NMR spectroscopy of urine samples in conjunction with statistical pattern recognition and compound identification allowed for the metabolic changes associated with TBP treatment to be identified. Discerning NMR spectral regions corresponding to three TBP metabolites, dibutyl phosphate (DBP), N-acetyl-(S-3-hydroxybutyl)-L-cysteine and N-acetyl-(S-3-oxobutyl)-L-cysteine, were identified in TBP-treated rats. In addition, the 1H NMR spectra revealed TBP-induced variations of endogenous urinary metabolites including benzoate, urea, and trigonelline along with metabolites involved in the Krebs cycle including citrate, cis-aconitate, trans-aconitate, 2-oxoglutarate, succinate, and fumarate. These findings indicate that TBP induces a disturbance to the Krebs cycle energy metabolism and provides a biomarker signature of TBP exposure. The three metabolites of TBP, dibutylphosphate, N-acetyl-(S-3-hydroxybutyl)-L-cysteine and N-acetyl-(S-3-oxobutyl)-L-cysteine, which are not present in the control groups, are the most important factors in separating the TBP and control groups (p<0.0023), while the endogenous compounds 2-oxoglutarate, benzoate, fumarate, trigonelline, and cis-aconitate were also important (p<0.01).
References
[1]. Neerathilingam, M.; Volk, D. E.; Sarkar, S.; Alam, T. M.; Alam, M. K.; Ansari, G. A. S.; Luxon, B. A., 1H NMR-based metabonomic investigation of tributyl phosphate exposure in rats. Toxicology Letters 2010, 199 (1), 10-16.
You may like
Related articles And Qustion
Lastest Price from Tributyl phosphate manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 126-73-8
- Min. Order:
- 200KG
- Purity:
- 99%min
- Supply Ability:
- 100tons/month

US $10.00/KG2025-04-21
- CAS:
- 126-73-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt