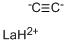What is the use and crystal structure of Lanthanum carbide?
Lanthanum carbide (LaC2) is a chemical compound. It is being studied about the manufacture of certain types of superconductors and nanotubes.
Properties
Lanthanum carbide reacts with water to form acetylene, C2H2, and a mixture of complex hydrocarbons.LaC2 is a metallic conductor, in contrast to CaC2 which is an insulator.
Crystal structure
The crystal structure of Lanthanum carbide shows that it contains C2 units with a C-C bond length of 130.3 pm, which is longer than the C-C bond length in calcium carbide, 119.2 pm, which is close to that of ethyne.
The structure of LaC2 can be described as La3+C22−(e-) where the electron enters the conduction band and antibonding orbitals on the C2 anion, increasing the bond length. This is analogous to the bonding present in the nitridoborate, CaNiBN.
Uses
Lanthanum Carbide is used to study the manufacture of certain types of superconductors and nanotubes
Safety
TSCA Status: Listed. A flammable solid.