What is the use and hazards of Samarium oxide?
Samarium oxide is commonly white to off-yellow in color and is often encountered as a highly fine dust-like powder. It readily forms on the surface of samarium metal under humid conditions or temperatures over 150°C in dry air.
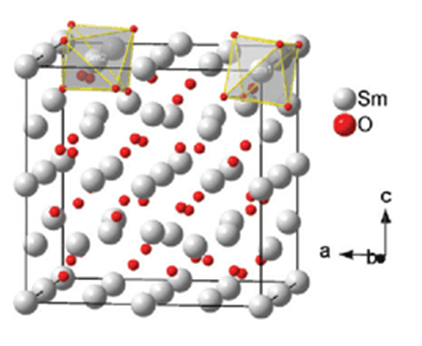
Crystal structure
Samarium oxide has a cubic structure.
Reactions
Samarium oxide dissolves in mineral acids, forming salts upon evaporation and crystallization:
Sm2O3 + 6 HCl → 2 SmCl3 + 3 H2O
The oxide can be reduced to metallic samarium by heating with a reducing agent, such as hydrogen or carbon monoxide, at elevated temperatures.
Uses
Samarium oxide is used in optical and infrared-absorbing glass to absorb infrared radiation. Also, it is used as a neutron absorber in control rods for nuclear power reactors. Samarium oxide catalyzes the dehydration and dehydrogenation of primary and secondary alcohols. Another use involves the preparation of other samarium salts.
Health hazards
Inhalation:May cause writhing, ataxia, labored respiration, walking on the toes with arched back, sedation, pneumoconiosis, hemoglobinemia, and lung granulomas.
Ingestion:May act as a blood anticoagulant.
Skin:No chronic health effects recorded.
Eye:No chronic health effects recorded.
Target Organs: These may affect the blood and lungs.
You may like
See also
Lastest Price from Samarium oxide manufacturers

US $4.00-3500.00/kg2025-06-09
- CAS:
- 12060-58-1
- Min. Order:
- 1000kg
- Purity:
- 99.5%
- Supply Ability:
- 20MT

US $0.00-0.00/KG2025-04-15
- CAS:
- 12060-58-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg