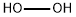What is the pH of hydrogen peroxide?
Mar 5,2024
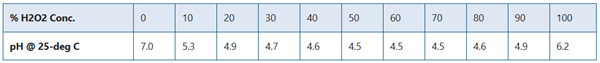
The pH of hydrogen peroxide is usually between 3 and 6, depending on concentration and temperature. It is usually about 4.5. The addition of acid increases the acidity to a pH of about 3.5, thereby increasing stability. Peroxide must remain at an acidic pH until it is mixed with an alkaline hair dye. The alkalinity of the hair dye triggers the breakdown of the peroxide, which forms the dye in the colour and lightens the natural hair colour. It is a very weak acid and can form hydroperoxides or peroxide salts or derivatives of many metals. The change in pH with peroxide concentration is shown in the chart below:
You may like
What are the pathways for glucose synthesis?
Dec 17, 2025