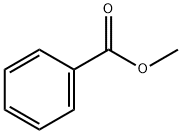
What is the nitration process of Methyl benzoate?
Methyl m-nitrobenzoate (methyl 3-nitrobenzoate) is an organic synthesis intermediate. It can be used as a starting material for the preparation of dyes and crop agents. Currently, it is obtained by nitrating methyl benzoate with mixed acid 2 (an aqueous mixture of nitric acid and sulfuric acid). Nitration is an exothermic reaction and, like polymerization, is one of the most dangerous reactions at the industrial level.
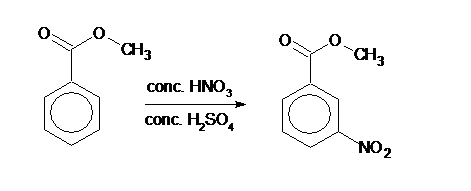
The synthetic reaction steps of methyl 3-nitrobenzoate are as follows:
Weigh 2.0 g of methyl benzoate into a dry conical flask, slowly swirl 4 cm3 of concentrated sulfuric acid into the methyl benzoate, mix thoroughly and cool in an ice bath. Then transfer 1.5 cm3 of concentrated nitric acid to a dry test tube. Immerse the nitric acid partially in an ice-water bath to cool, then slowly add 1.5 cm3 of concentrated sulfuric acid, mix and shake well, and cool. Get a nitration mixture. Finally, slowly add the nitration mixture into a conical flask while stirring (keep the temperature below 6 °C). After standing at room temperature for 15 minutes, add ice and filter, and recrystallize in hot ethanol to obtain the product.
References:
[1] ILARIA DI SOMMA*. Kinetic and Safety Characterization of the Nitration Process of Methyl Benzoate in Mixed Acid[J]. Organic Process Research & Development, 2012, 16 12: 1877-2064. DOI:10.1021/op300043x.Related articles And Qustion
See also
Lastest Price from Methyl benzoate manufacturers

US $10.00/kg2025-04-21
- CAS:
- 93-58-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/KG2025-03-07
- CAS:
- 93-58-3
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000kg