What is the Ionic Charge of Copper?
In its ionic state, copper (Cu) can have either a +1 or a +2 charge. Copper is a transition metal, which means it can exhibit variable valency and form ions with partially filled d orbitals.
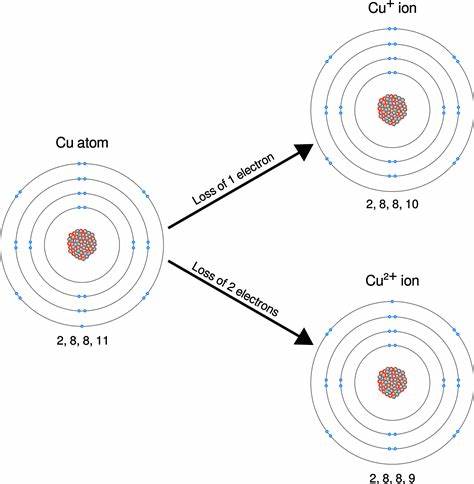
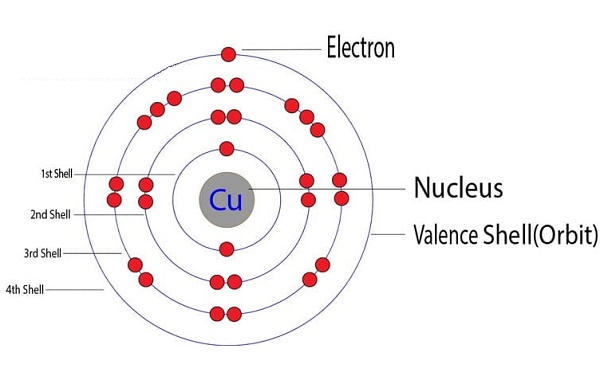
A neutral copper atom has an atomic number of 29 and a total of 29 electrons. Its electron configuration is [Ar]3d¹⁰4s¹. When copper loses one electron from its 4s orbital, it forms the copper(I) ion (Cu⁺) with a +1 charge and an electron configuration of [Ar]3d¹⁰.
On the other hand, copper can also lose two electrons, one from the 4s orbital and one from the 3d orbital. This results in the copper(II) ion (Cu²⁺) with a +2 charge and an electron configuration of [Ar]3d⁹.
Therefore, the charge of a copper ion can be either +1 or +2, depending on the number of electrons it loses.
You may like
Related articles And Qustion
See also
Lastest Price from Copper manufacturers

US $0.00-0.00/kg2025-12-09
- CAS:
- 7440-50-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $6.00/kg2025-04-21
- CAS:
- 7440-50-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month