Environmental Fate of Copper
Copper has long been used by humans for a variety of reasons. The name copper derives from the Latin for the metal, cuprum, which is named for the Roman source, the island of Cyprus. Copper has been used in a variety of alloys; of particular importance among copper alloys is bronze, which comprised most of the tools and weapons of the age that bears its name. Brass, a copper–zinc alloy, is also highly used, for example, in brass musical instruments. Copper has also long been used as a building material, and owing to the metal’s malleability, as well as high thermal and electric conductivity, continues to find new uses.

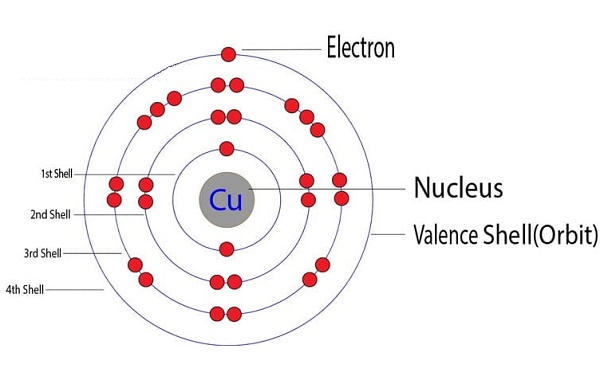
Copper and its compounds are naturally present in the earth’s crust. Natural discharges to air and water may be significant. Therefore, it is important to consider the background levels that are commonly found and distinguish these from high levels that may be found as a result of anthropogenic activity. Copper is emitted into the air naturally from windblown dust, volcanoes, and anthropogenic sources, the largest of which are being primary copper smelters and ore processing facilities. It is associated with particulate matter. The mean concentration of copper in the atmosphere is 5–200 ng m-3.
Uses
Copper is an essential trace element. Adequate daily requirements
are 2–3 mg day1. It is widely distributed in nature and
extensively used in industry. It is used as an electrical
conductor, as a component in a variety of alloys (including
gold and silver alloys), and as a constituent in paints and
ceramic glazes. Because it corrodes at a very slow rate, it is used
extensively for water pipes. In addition, copper sulfate mixed
with lime is used as a fungicide.
Medicinally, copper sulfate is used as an emetic. It has also
been used as an antihelminthic (antiparasitic agent) based on
its astringent and caustic actions.
Environmental Fate
The largest release of copper by far is to land, and the major sources of release are mining and milling operations, agriculture, solid waste, and sludge from publicly owned treatment works. Sediment is an important sink and reservoir for copper. In relatively clean sediment, the copper concentration is<50 ppm; polluted sediment may contain several thousand ppm of copper.
Copper is released to water as a result of natural weathering of soil and discharges from industries and sewage treatment plants. Copper compounds may also be intentionally applied to water to kill algae. Of special concern is copper that gets into drinking water from the water distribution system.
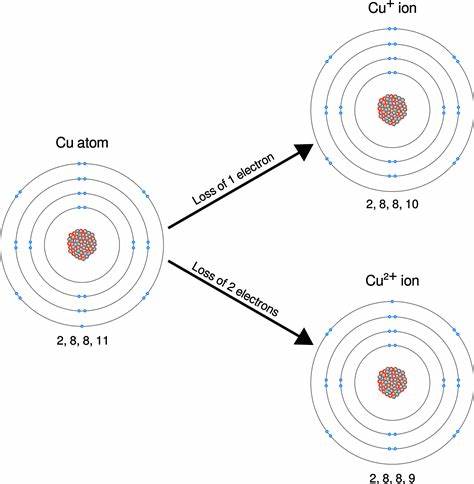
The major species of soluble copper found in freshwater,
seawater, and a combination of the two over a range of pHs is
Cu2+, Cu(HCO3)+, and Cu(OH)2. At the pH values and
carbonate concentrations characteristic of natural waters, most
dissolved Cu(II) exists as carbonate complexes rather than as
free (hydrated) cupric ions.
The transport of copper is largely dependent on source
characteristics as well as particle size; however, it can bind to
many inorganic ligands. Some copper compounds are water
soluble, and this can increase transport distance, as well as
likelihood the metal will be taken up by organisms or adsorb to
organic residues.
Mechanism
Copper reduces glutathione, which is necessary for normal cell viability. The amino acid transferases are inhibited in the presence of excess copper; lipid peroxidation also occurs. Copper combines with thiol groups, which reduces the oxidation state II to I in copper and oxidizes the thiol groups to disulfides, especially in the cell membrane.
You may like
Related articles And Qustion
Lastest Price from Copper manufacturers

US $0.00-0.00/kg2025-12-09
- CAS:
- 7440-50-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $6.00/kg2025-04-21
- CAS:
- 7440-50-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month