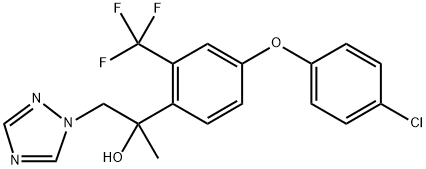
A novel fungicide-Mefentrifluconazole
Description
Mefentrifluconazole ([(2RS)− 2-[4-(4-chlorophenoxy)-α,α,α-trifluoro-o-tolyl]− 1-(1 H-1,2,4-triazol-1-yl)propan-2-ol]; MFZ) is the first marketed novel isopropanol triazole and is the youngest member of the “conazole” family of C14- demethylase inhibitors (DMIs), which block this vital enzyme within the fungal ergosterol biosynthesis pathway. This active ingredient combines potent broad-spectrum fungicidal efficacy, especially against cereal diseases, with a minimal likelihood of adverse side effects, such as developmental toxicity or endocrine disruption.
Uses
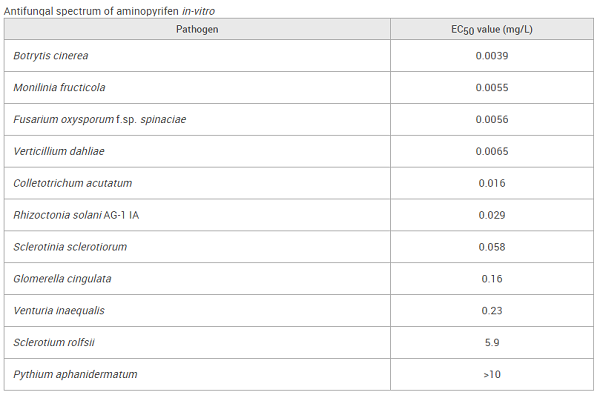
The compound inhibits sterol biosynthesis and alters the structure of the cell plasma membrane, thereby reducing cell viability. It acts by inhibiting fungal cytochrome P450-mediated 14α-demethylase activity and blocking ergosterol biosynthesis. It is highly effective for combating leaf spot, rust, and powdery mildew on cereals, soybeans, corn, vegetables, and fruit[1]. In particular, MFZ exhibits excellent efficiency against various fungal diseases on more than 60 crops[2]. Although cross-resistance to MFZ was confirmed in isolates exhibiting resistance to other triazole fungicides, mefentrifluconazole is still a promising fungicide for controlling fungal disease due to its favorable toxicity profile. MFZ has obtained registration in the United States, the European Union, Canada, Australia, Korea, and China. Mefentrifluconazole is currently registered for use against grape anthracnose, northern corn leaf blight, tomato leaf blight, and apple brown spot in China.
Synthesis method
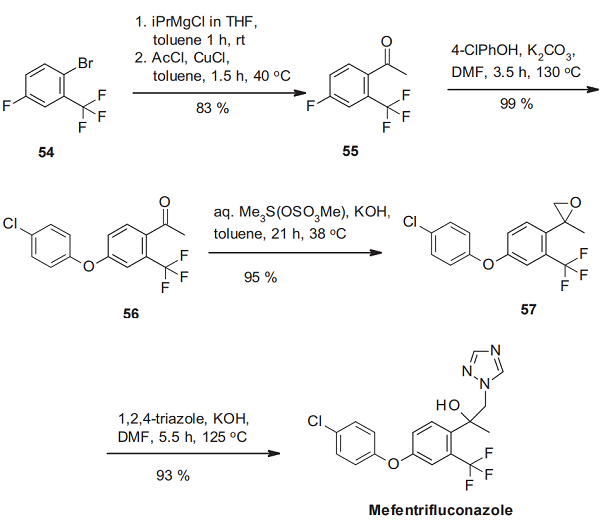
The synthesis of mefentrifluconazole starts with the acetylation of 2-bromo-5-fluorobenzotrifluoride (54) via its Grignard intermediate to the acetophenone derivative 55[3]. The introduction of a 4- chlorophenoxy moiety by nucleophilic aromatic substitution leads to the phenoxyphenyl derivative 56. The ketone function is then transformed into an epoxide by the Corey-Chaykovsky reaction using trimethyl sulfonium-methylsulfate in the presence of potassium hydroxide. Ring-opening of the oxirane function of the resulting 57 with 1,2,4-triazole affords mefentrifluconazole.
References
[1] Yangyang Gao. “Activity of the Novel Fungicide Mefentrifluconazole Against Colletotrichum scovillei.” Plant disease 105 5 (2021): 1522–1530.
[2] Feng Cui. “Toxicity of mefentrifluconazole enantiomers on multiple stages of zebrafish (Danio rerio).” Journal of Environmental Chemical Engineering 96 1 (2022).
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.