Aminopyrifen: Biological activity and Synthesis method
Description
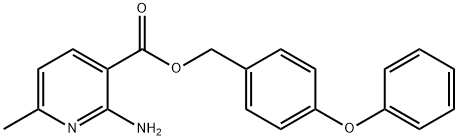
Aminopyrifen, 4-phenoxy benzyl 2-amino-6-methylnicotinate, is a 2-amino nicotinate discovered by Agro-Kanesho with development code AKD-5195. Aminopyrifen shows strong antifungal activity against various plant pathogenic fungi, including B. cinerea (WO2014006945, JP2016199526A). It has been reported to block the GWT-1 protein in fungal glycosylphosphatidylinositol(GPI)-anchor biosynthesis and, therefore, possesses a mode of action entirely novel to the fungicide market. As GPI-anchored proteins are essential for maintaining cell wall integrity, inhibiting their biosynthesis leads to the perturbation of mycelial growth[1]. In a preliminary study, this fungicide did not show cross-resistance to benzimidazole, dicarboximide, anilinopyrimidine, DMI, QoI, and SDHI, suggesting its mode of action may be unique.
Biological activity
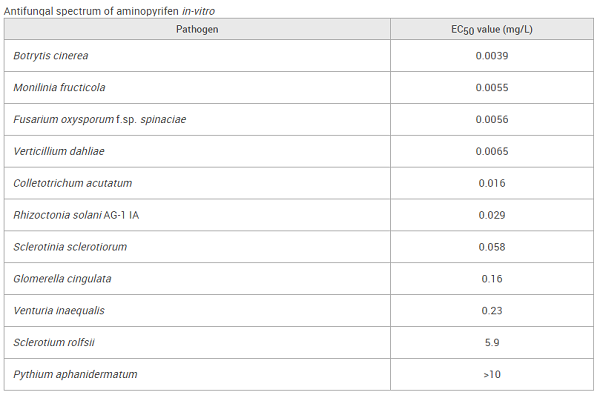
On potato dextrose agar medium containing aminopyrifen, this fungicide showed high activity against Ascomycetes and its related anamorphic fungi: Botrytis cinerea, Colletotrichum acutatum, Fusarium oxysporum f. sp. lycopersici, Glomerella cingulata, Monilinia fructicola, Sclerotinia sclerotiorum, Venturia inaequalis, and Verticillium dahliae (EC50: 0.0039–0.23 mg/L, shown below). Regarding Basidiomycetes, while aminopyrifen was highly effective against Rhizoctonia solani AG-1 IA (EC50: 0.029 mg/L), it showed low activity against Sclerotium rolfsii (EC50: 5.9 mg/L)[2]. In contrast, this fungicide at 10 mg/L did not exhibit activity against pseudofungi, e.g., Pythium aphanidermatum, which belongs to Oomycetes. In potted tests, the fungicide had no activity against tomato late blight (Phytophthora infestans). However, it exhibited activity against cucumber anthracnose (Colletotrichum orbiculare) and rice blast (Pyricularia oryzae) at EC50 3.6 mg/L and 1.2 mg/L, respectively.
Synthesis method
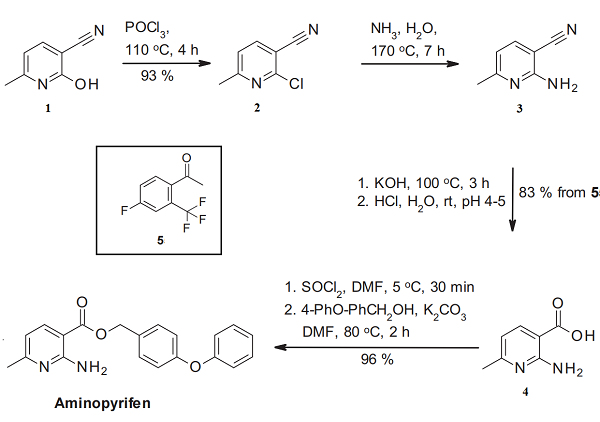
The synthesis of aminopyridine starts with the phosphorus oxychloride-mediated chlorination of the nicotinonitrile derivative 1[3]. The resulting trisubstituted pyridine 2 is converted into the aza anthranilic acid equivalent 4 by amination and nitrile hydrolysis. Finally, converting the nicotinic acid derivative 4 into the corresponding acid chloride and its esterification with 4-phenoxy benzyl alcohol leads to aminopyrifen.
References
[1] Masahiro Hatamoto . “A novel fungicide aminopyrifen inhibits GWT-1 protein in glycosylphosphatidylinositol-anchor biosynthesis in Neurospora crassa.” Pesticide Biochemistry and Physiology 156 (2019): Pages 1-8.
[2] Masahiro Hatamoto. “Aminopyrifen, a novel 2-aminonicotinate fungicide with a unique effect and broad-spectrum activity against plant pathogenic fungi.” Journal of Pesticide Science 46 2 (2021): 198–205.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.